Dextromethorphan
| Summary sheet: Dextromethorphan |
| Dextromethorphan | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | DXM, DMO, DM, Dex, Robitussin, Delsym, DexAlone, Duract | ||||||||||||||||||||||||||||||||
| Substitutive name | Dextromethorphan | ||||||||||||||||||||||||||||||||
| Systematic name | (4bS,8aR,9S)-3-Methoxy-11-methyl-6,7,8,8a,9,10-hexahydro-5H-9,4b-(epiminoethano)phenanthrene | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Dissociative | ||||||||||||||||||||||||||||||||
| Chemical class | Morphinan | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
| Benzodiazepines | |||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||
| Bupropion | |||||||||||||||||||||||||||||||||
| ΑMT | |||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||
| SSRIs | |||||||||||||||||||||||||||||||||
| Antihistamines | |||||||||||||||||||||||||||||||||
Dextromethorphan (also known as robo, dex, DM, and DXM) is a dissociative substance of the morphinan class. It is the primary active ingredient in many common over-the-counter (OTC) cold and cough medicines. When exceeding approved doses, DXM produces dissociative effects similar to those of ketamine and phencyclidine (PCP). It acts as a noncompetitive NMDA receptor antagonist [1].
DXM was first reported in 1953 and approved for use as a cough suppressant in the United States in 1958.[2] After its approval, it was introduced as an OTC medication under the name Romilar. As early as 1975, the popularity and extensive abuse of DXM was recognized, and Romilar was voluntarily removed from the OTC market.[3] A few years later, companies began introducing various refined DXM products designed to deter abuse, such as including ingredients with an unpleasant taste.
However, recreational use of DXM has persisted and is a considered a growing trend, particularly among teenagers seeking low cost and easily available highs.[4]
Subjective effects include dissociation, time distortion, bodily hallucinations, immersion enhancement, motor control loss, euphoria, and ego loss. Users commonly describe low doses as producing an alcohol-like intoxication while higher doses produce effects similar to ketamine or PCP. It is often reported to produce a strong, uncomfortable body load with significant nausea.
The effects and tolerability of DXM are highly variable between users which may be due to individual differences in the genes underlying metabolism. As a result, many users find the experience of DXM to be either unpleasant, neutral, or dull, while others report mystical-like psychedelic experiences.
It should be noted that DXM in freebase form (as found in Robocough RoboTablets) is around 27-37% more potent than its hydrobromide form due to a higher concentration of DXM by weight. One should take this into account when calculating their dose to avoid a potential overdose.
The toxicity of DXM in recreational doses is unclear and has been the subject of controversy. There is some evidence that suggests NMDA antagonists may have neurotoxic effects when used in excess. Many cases of DXM dependence and abuse have been documented.[citation needed] It is strongly advised to use harm reduction practices if using this substance.
History and culture
The racemic parent compound of dextromethorphan, racemethorphan, was first described in a Swiss and US patent application from Hoffmann-La Roche in 1946 and 1947, respectively. A patent was granted in 1950. A resolution of the two isomers of racemethorphan with tartaric acid was published in 1952,[5] and DXM was successfully tested in 1954 as part of US Navy and CIA-funded research on nonaddictive substitutes for codeine.[6]
DXM was approved by the FDA in 1958 as an over-the-counter antitussive, or cough suppressant.[5] As had been initially hoped, DXM was a solution for some of the problems associated with the use of codeine phosphate as a cough suppressant, such as sedation and opiate dependence, but like the dissociative anesthetics phencyclidine and ketamine, DXM later became associated with nonmedical use.[3][5]
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse, and was replaced by cough syrup in an attempt to cut down on abuse.[3]
The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about DXM, and online discussion groups formed around use and acquisition of the substance.
As early as 1996, DXM HBr powder could be purchased in bulk from online retailers, allowing users to avoid consuming DXM in syrup preparations.[5] As of January 1, 2012, dextromethorphan is prohibited for sale to minors in the state of California, except with a doctor's prescription.[7]
Chemistry
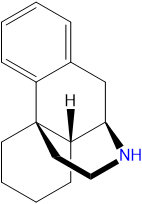
Dextromethorphan is a dextrorotatory molecule of the morphinan class. It contains a phenanthrene core structure with one aromatic ring (benzene) bound to two saturated rings (cyclohexane). Additionally, it contains a saturated piperidine ring attached to R9 and R13 of the core structure. DXM is substituted at RN with a methyl group and at R3 with a methoxy group.
Pharmacology
The pharmacology of DXM is not completely understood. In vitro studies suggest that the primary mechanism of action of DXM is blockade of N-methyl-D-aspartate (NMDA) receptors. NMDA receptors are a type of glutamate receptor; glutamate is the primary excitatory neurotransmitter. Blockade of NMDA receptors therefore interferes with excitatory signaling in the central nervous system. This mechanism of action is similar to ketamine and PCP.
Rather than acting as a direct NMDA receptor antagonist itself, dextromethorphan acts as a prodrug of its much more potent metabolite dextrorphan, and this is the actual mediator of its dissociative effects.
Additional pharmacological mechanisms include actions as a nonselective serotonin reuptake inhibitor[8], alpha-3 beta-4 nicotinic receptor antagonist[9] and a sigma-1 receptor agonist.[10][11]
At high doses, DXM can cause an increase in systolic and diastolic blood pressure along with an increase in heart rate.[12] DXM also increases blood plasma levels of adrenocorticotropic hormone (ACTH) and corticosterone.[13]
Although DXM is a morphine derivative, it isn't a strong μ-opioid agonist unlike most compounds in that class, such as heroin and codeine.
| Binding Sites | Binding Affinity Ki (nM)[14] |
|---|---|
| NMDA | 8945 |
| Sigma-1 | 138 |
| SERT | 40 |
| NET | 240 |
| μ-opioid | 1280 |
| κ-opioid | 7000 |
| δ-opioid | 11500 |
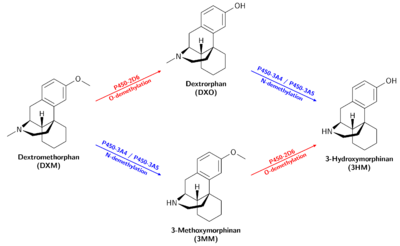
Metabolism
DXM is O-demethylated into Dextrorphan (DXO / D-3-hydroxy-N-methylmorphinan) by the CYP2D6 enzyme.[15][16] DXM is also N-demethylated into 3-methoxymorphinan (MEM / Morphinan) by the CYP3A4 enzyme[16][17] and to a lesser extent CYP3A5.[18]
Dextrorphan and 3-methoxymorphinan are both metabolized into 3-hydroxymorphinan. Dextrorphan is N-demethylated by CYP3A4 and 3-Methoxymorphinan is O-demethylated by CYP2D6. CYP2D6 O-demethylation is more effective than CYP3A4 N-demethylation.[16]
Variations of how individual persons metabolize DXM may significantly alter the nature of the experience. Poor metabolizers clear less DXM than the average person, leading to a higher ratio of DXM to DXO in the bloodstream[19], as well as overall increased potency and duration, due to less lost to the largely inactive metabolites 3MM and 3HM. CYP2D6 and CYP3A4 inhibitors have a similar effect.
Dextrorphan
Dextrorphan is produced by O-demethylation of dextromethorphan through the CYP2D6 enzyme and contributes to the psychoactive effects of dextromethorphan.[20] It is pharmacologically similar to that of dextromethorphan (DXM); however, it is much more potent as an NMDA receptor antagonist[14] as well as much less active as a selective serotonin reuptake inhibitor.[13]
It is also about 3-fold less potent of a α3β4 nicotinic receptor antagonist than DXM[9] and has a lower affinity for sigma-1 receptors.[10]
| Binding Sites | Binding Affinity Ki (nM)[14] |
|---|---|
| NMDA | 486 |
| Sigma-1 | 351 |
| SERT | 484 |
| NET | 340 |
| μ-opioid | 420 |
| κ-opioid | 5950 |
| δ-opioid | 34700 |
3-Methoxymorphinan
3-Methoxymorphinan (also known as 3MM) is produced by the N-demethylation of dextromethorphan by the CYP3A4 enzyme[16] and inhibits the CYP2D6 enzyme.[21] It has local anaesthetic effects.[22]
3-Hydroxymorphinan
3-Hydroxymorphinan (also known as 3HM) is produced by O-demethylation of 3-methoxymorphinan by CYP2D6 and metabolization of dextrorphan by CYP3A4 and CYP3A5[23]. 3-Hydroxymorphinan exhibits neuroprotective and neurotrophic effects.[24][25][26]
Subjective effects
The head space of DXM is often described as distinctly hallucinogenic, impairing, disorientating and generally less clear-headed in comparison to that of MXE and ketamine.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation[12] & Sedation - At lower, recreational doses, DXM is predominantly stimulating. However, it can produce waves of tiredness, or the desire to lay down with the eyes closed in a sleep-like state. As higher dosages are approached, the experience generally turns very sedating and lethargic, sometimes resulting in the user not moving at all.
- Perception of bodily lightness - This creates the sensation that the body is floating and has become entirely weightless. This effect is strangely stimulating and encourages physical activities at low to moderate dosages by making the body feel light and effortless to move.
- Bodily control enhancement - This effect manifests inconsistently at lower dosages but can be quite noticeable. At the higher plateaus, it gives way to motor control loss, gait alteration, and spatial disorientation.
- Spontaneous bodily sensations - The DXM "body high" is a sharp, pleasurable and warm tingling sensation which can be localized to the hands, feet, and head. In lower doses, it can produce an empowering stimulated sensation, at higher dosages a slight to heavy body load.
- Physical euphoria - This results in feelings of physical euphoria which range between mild pleasure to powerful, all-encompassing bliss.
- Appetite suppression - Appetite suppression on DXM is very strong and may persist through the next day.
- Changes in felt bodily form - This becomes quite prominent at moderate or high doses.
- Motor control loss[12] - A loss of gross and fine motor control alongside balance and coordination is prominent within DXM and becomes especially strong at higher dosages. One should be sitting down before the onset to prevent falling over and becoming injured.
- Spatial disorientation[12] - A spinning sensation is commonly felt that can result in mild disorientation as if one were falling through a hole.
- Nausea[12] - DXM can sometimes produce extreme nausea and vomiting, typically during the come up phase of the experience. This is more intense and consistent than the nausea produced by ketamine and MXE. This is likely not caused just by DXM itself, but rather by the medium which contains the DXM, which is usually syrup or gelatin capules. When pure DXM is consumed it is uncommon to experience severe nausea.
- Temperature regulation suppression
- Increased bodily temperature - Since dextromethorphan is a moderately potent serotonin reuptake inhibitor, it increases body temperature slightly at moderate to high doses.
- Increased blood pressure[12]
- Increased heart rate[12]
- Increased perspiration - This is the result of a combination of increased bodily temperature and temperature regulation suppression.
- Muscle spasms
- Orgasm suppression
- Decreased libido
- Difficulty urinating - This becomes more apparent with escalating doses and, in high doses, can result in a complete inability to urinate.
- Itchiness - This effect is colloquially known as "robo-itch". Many users never experience this effect while some individuals can experience it quite intensely. It is caused by histamine release and typically occurs as a relative factor of dosage.[citation needed]
- Cough suppression - Since dextromethorphan is a commonly sold cough medicine, it will have the same effect in recreational doses.
- Pain relief
- Muscle relaxation - This effect is inconsistent, and may alternate with bouts of muscle twitching.
- Optical sliding
- Dizziness - At higher dosages this can result in incapability to willingly stand up.
- Gustatory hallucination
- Physical autonomy - At very high dosages some may find to awake in strange locations, sometimes while standing or performing actions with no recollection of events or how they got there.
- Tactile suppression - This partially to entirely suppresses sense of touch, creating feelings of numbness within the extremities. It is responsible for the anesthetic properties of this substance.
- Pupil dilation- Pupil dilation is very noticeable at moderate to high doses but is less prevalent at low doses in the 1st plateau.
- Gait alteration - This is a common effect on DXM and is commonly referred to as "robo walking". As with itchiness, some users may never experience this effect while others may experience it quite intensely.
Visual effects 
-
Enhancements
- Colour enhancement - Tends to be less rich, vivid, and saturated than the colour enhancement experienced on psychedelics, and often tends to change colors as much as intensify them - a red, orange, or violet visual tinting is frequently present at higher dosages.
- Magnification - This effect is uncommon and typically occurs in conjunction with perspective distortion.
- Peripheral vision enhancement - This effect usually only occurs at low doses.
- Frame rate enhancement - This effect is rare and occasionally experienced on the lower plateaus. It appears to be setting dependent.
Suppression
- Double vision - This component is prevalent at moderate to heavy dosages and makes reading impossible unless one closes an eye.
- Pattern recognition suppression - This effect generally occurs at higher dosages and makes one unable to recognize and interpret perceivable visual data. Examples can include an inability to recognize faces or motion.
- Frame rate suppression - This is the so-called "flanging" effect, which at appropriately high doses can affect sight, sound and at higher levels phrases, faces and thinking.
- Nystagmus - At very high dosages one may be incapable of recognizing things such as movement of objects or human faces.
- Visual acuity suppression - Vision is reported to be very dream-like, frequently with a hazy or "static-like" overlay affecting the visual field.
Distortions
- After images - Some users report seeing their surroundings for a few seconds after closing their eyes.
- Depth perception distortions
- Drifting - Visual drifting has been reported to occur on DXM, although it is uncommon. This effect is unrealistic in appearance. The distortions fast and smooth in motion, and are fleeting in appearance.
- Environmental cubism
- Environmental orbism
- Perspective distortions
- Scenery slicing
- Tracers
- Visual haze
- Visual stretching
Geometry
The visual geometry produced by DXM can be described as very bright, colorful, psychedelic and intricate when compared to that of ketamine or MXE, but darker than psychedelics like LSD. There are moments where the perceived geometry may start to slow down, wobble or freeze, which is not usually seen with psychedelics or dissociatives. Spontaneously they can transform into a hallucinatory state with the eyes closed.It does not extend beyond level 4 and can be comprehensively described through its variations as intricate in complexity, algorithmic in style, synthetic in feel, unstructured in organization, brightly lit in lighting, multicolored in scheme, glossy in shading, soft in edges, small in size, slow in speed, equally smooth and jittery in motion, equal in rounded and angular corners, immersive in-depth and consistent in intensity.
Hallucinatory states
At high dosages, DXM can produce a full range of high level hallucinatory states in a fashion that is less consistent and reproducible than that of many other commonly used psychedelics. These effects include:
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - In comparison to other dissociatives, this effect can occur at heavy dosages, but is extremely infrequent in comparison to the same effect found within deliriants. It can be comprehensively described through its variations as delirious in believability, autonomous in controllability and solid in style. The most common theme for this effect to follow is one of experiencing and talking to friends around oneself when they are not actually present.
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - In comparison to other dissociatives, this effect can occur at heavy dosages, but is less common than the same effect found within psychedelics and deliriants, although can sometimes become very encompassing nonetheless. It can be comprehensively described through its variations as delirious in believability, fixed in style, equal in new experiences and memory replays in content, autonomous in controllability and solid in style.
Cognitive effects 
-
- Analysis suppression
- Anxiety suppression or Anxiety - Although DXM typically suppresses anxiety, it is also able to produce it in certain conditions. Panic attacks can occur at heavy dosages.
- Conceptual thinking
- Confusion[12] - This effect almost represents delirium in 3rd plateaus, although, your cognitive thinking abilities start to deminish when this effect starts with depersonalization.
- Cognitive euphoria - While states of cognitive euphoria are commonly reported, this effect can unpredictably manifest itself as cognitive dysphoria for no apparent reason, particularly at higher doses.
- Cognitive fatigue[12]
- Creativity enhancement
- Decreased libido
- Déjà vu
- Delusion - This effect can occur spontaneously among some users and is more likely to occur at higher doses.
- Depersonalization - This effect only begins to show in high 2nd / 3rd / 4th plateaus.
- Derealization
- Disinhibition - This effect can be quite significant even at moderate dosages.
- Ego inflation
- Dream potentiation
- Emotion enhancement - Though this effect isn't as consistent as it is with other commonly used hallucinogens it is more prominent than with most dissociatives.
- Empathy, affection, and sociability enhancement - This is commonly reported as being similar to but less prominent than with other commonly used entactogens such as MDMA or MDA.
- Immersion enhancement
- Increased sense of humor - Mostly present below the upper plateaus.
- Increased libido - This effect is exclusively felt in low doses.
- Increased music appreciation - This effect can be very intense with DXM, especially at the lower plateaus. Listening to music greatly intensifies the experience and produces strong euphoria.
- Memory suppression
- Amnesia - This effect is usually only present with higher dosages, past the threshold for ego death (which can vary between people based on their individual metabolism). It can range from partial to complete memory loss of the experience. Generally, more dull parts of the experience tend to not be remembered at all. Frequent use can sometimes contribute to this, even for days after the experience.
- Focus suppression[12]
- Motivation suppression[12]
- Novelty enhancement
- Personal bias suppression - This effect is not usually as pronounced as it is with other more commonly used hallucinogens such as LSD or psilocin.
- Personal meaning enhancement - This effect is typically only present on the lower plateaus and varies in its believability and content.
- Thought deceleration
- Time distortion - Time while on DXM often feels very stretched out. For example, it may feel like hours have passed when in reality only ten minutes have passed. There also seems to be a general difficulty of what time certain events took place.
- Sleepiness - This effect peaks after the first hour of the initial dose taken, once you reach peak effects, it slowly dies down making you awake, yet asleep.
Auditory effects 
Disconnective effects 
-
- Cognitive disconnection
- Physical disconnection - Although this effect is present, it is usually not as powerful or as consistent as with ketamine or PCP.
- Visual disconnection - This eventually results in the DXM's equivalent of the "K-hole" or more specifically, holes, spaces and voids alongside of structures.
Multi-sensory effects 
-
- Synaesthesia - In its fullest manifestation, this is a very rare and non-reproducible effect. Increasing the dosage can increase the likelihood of this occurring, but seems only to be a prominent part of the experience among those who are already predisposed to synaesthetic states.
Transpersonal effects 
Afterglow 
-
The afterglow is a feeling that can occur in the following day or weeks after the experience. It can be described regarding its physical sensation as one of euphoria, rejuvenation, relaxation and a light bounciness.
Cognitive manifestations include a loss of anxiety, feelings of content, increased music appreciation and other sensory stimuli that are sometimes accompanied with mild derealization or depersonalization.
Plateaus
The online DXM community categorizes the types of experiences that can result from oral DXM use into five "plateaus" which are characterized by qualitatively distinct effects.
First Plateau (1.5 - 2.5 mg/kg) - The effects felt in the first plateau are usually not very intense. They can include but are not limited to: cognitive euphoria, increased music appreciation, time distortion, pupil dilation, and stimulation. First plateau is often described as a "drunk" feeling.
Second Plateau (2.5 - 7.5 mg/kg) - Many DXM users consider this the to be the most recreational plateau. The second plateau is more sedating than stimulating, while euphoria and visual disconnection are more intense. Additional effects of the second plateau can include but are not limited to: wakefulness, physical euphoria, spatial disorientation, perception of bodily lightness, frame rate suppression, and increased music appreciation. Many users of DXM do not proceed past the second plateau, as the desired effects are thought to be outweighed by the increasing adverse effects, including a more pronounced "body load". Music is often reported to be greatly enhanced at this plateau, described as rich, clear, and amplified.
Third Plateau (7.5 - 15 mg/kg) - The Third Plateau is reported to be much more intense than the second, and should be gradually worked up towards. The effects of the third plateau can include but are not limited to: dissociation, sedation, nausea (during comeup), memory suppression and ego death, auditory hallucinations, internal hallucinations, cognitive dysphoria, euphoria, anxiety, delusions, and all the effects of the second plateau. Third plateau trips have been described as euphoric and profound by some users.
Some users have described an omnipotent feeling that one is in control of the entire universe, accompanied with CEVs. Closed eye visuals are often present, consisting of usually darker colored geometric patterns, vast landscapes, and scenes. Music can greatly enhance the effect, although it can also sound tinny and distorted. Users have reported being able to break down every single fragment of a song and visualize all of it in their minds; reliving past memories has also been reported.
The third plateau is often reported to be extremely introspective and a number of users describe being inspired to improve their lives. The afterglow is reported as tiring, but a mood boost is often reported for days or weeks afterward.
Fourth Plateau (15 - 20 mg/kg) - The effects of the fourth plateau can include but are not limited to external hallucinations, complete dissociation, and all the effects of the third plateau, with greater intensity. Amnesia can occur, leading to memory blackouts or brownouts.
Doses of DXM in this range are very dangerous and have a high risk of injury and overdose, and are therefore advised against.
Fifth Plateau (also known as "Plateau Sigma") -
Plateau Sigma is neurotoxic and has high potential to cause serotonin syndrome, it is therefore strongly advised against.
A common method to reach "Plateau Sigma" is to take a second plateau dose, followed by another second plateau dose three hours later, then take a fourth plateau dose upon the peak of the second dose. Plateau sigma usually results in delirious hallucinations, dysphoria, delirium, psychosis, and anxiety; plateau sigma's subjective experience is therefore usually very unpleasant and unpredictable. Separate dosages are needed to achieve plateau sigma because the initial dosages pre-occupy the hepatic enzymes that convert DXM to Dextorphan, leading to a higher blood concentration of un-converted DXM (followed by its own subjective effects) and a longer process for it to clear from the body. Due to this enzymatic effect, plateau sigma can accidentally occur due to redosages, drug/medication interactions (i.e substrates for CYP2D6), and/or enzyme deficiency/liver dysfunction.[27] Due to the variance in how each individual clears DXM from the body, the subjective experience of plateau sigma can potentially last from one to four days.
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience: 300mg DXM (Oral) - Brink of the third
- Experience: 300mg DXM + 150mg Bupropion (Oral) - It was good night
- Experience: 450mg DXM + THC - A shallow dive into the 3rd plateau
- Experience:100/100/100mg, first time with it
- Experience:1000mg / 1200mg / 1400mg / 1600mg - heroic doses
- Experience:1064mgs - Fascinating DXM experience - Unusual effects
- Experience:110mg Diphenidine (vaporized) + 354mg DXM - instant ego death
- Experience:110mg Diphenidine (vaporized) + 354mg DXM- instant ego death
- Experience:250mg DXM (oral) - Fun but Underwhelming
- Experience:250mg DXM - DXM Itch and Trip Report
- Experience:300mg DPH + 600mg DXM - An Interesting Combo
- Experience:300mg DXM + 25mg DMT + Cannabis - A crazy night
- Experience:350mg DXM - A surprisingly profound experience
- Experience:354mg DXM, weed, nicotine - Descending into the void
- Experience:400mg DXM + 300mg DPH – Bacterial friends
- Experience:535mg - My First DXM Trip
- Experience:700mg - To the dextroverse.
- Experience:750mg - Experiencing Void; Dissociation Of Reality And Self
- Experience:An intense slap: 60 μg LSD and 280 mg DXM
- Experience:DXM & DPH in combination
- Experience:DXM (125 mg) - Untitled
- Experience:DXM (1500 mg, oral) - "Who are you? I, am nobody."
- Experience:DXM (225 mg, oral) - A Missed Opportunity
- Experience:DXM (340 mg) + DMT (30 mg, smoked) + Cannabis - Amazing Synergy
- Experience:DXM (660 mg) - Life Changing Experience
- Experience:DXM 1200mg - A calm flood.
- Experience:DXM and Cannabis: 100mg - Unexpected Strong Trip
- Experience:DXM polistirex (~650 mg, cough syrup) - Long euphoric trip
- Experience:Salvia divinorum (1 gram plain leaf, smoked. Combination with DXM, Nitrous, and Tobacco) - A journey into godhood with Diviner's Sage
- Experience:~6000mg DXM Freebase + 4g's Red Vein Kratom + HHC + Nitrous - Five Days of Disassociation
Additional experience reports can be found here:
Common usage
Available forms
DXM is available in several forms that can be found over the counter or online.
- Cough syrup is the most common form online and over the counter. Well-known brands include Benylin, DayQuil, Delsym, NyQuil, Robitussin, Omol, and Siltussin. Many of these products contain other medicines, including aspirin, acetaminophen, caffeine, guaifenesin or pseudoephedrine. Within the UK, Benylin dry coughs 7.5mg/5ml syrup (225mg dosage per 150ml bottle) is available behind the counter at every pharmacy. Generic brands are also available within these shops for a consistently lower price. In recent years in the United States, a DXM formulation called RoboCough (which has no other active ingredients, and comes in small bottles) has become popular.
- Gel capsules, lozenges, soft pastilles, and pills are available online and over the counter. Well-known brands include Benylin, Comtrex, Coricidin, DayQuil, Mucinex, NyQuil, and Robitussin. Many of these products contain other medicines, including aspirin, acetaminophen, caffeine, guaifenesin or pseudoephedrine.
- Pure powder is available online. This is the safest way to use DXM, as there is no danger of overdose from secondary chemicals. However, one should still test their powder to avoid ingesting toxic byproducts or a preparation falsely advertised as DXM powder.
It is important not to overdose on other active ingredients or a combination of them. Common ingredients also contained in cough syrup and pills include:
- Acetaminophen: also named Paracetamol or abbreviated as APAP, it is an analgesic (pain-reliving medication). The recommended dose of acetaminophen in adults is 650 to 1,000 mg every 4 to 6 hours, not to exceed 4,000 mg in a 24-hour period. Single doses over 150 mg/kg or 7.5 g in adults have been considered potentially toxic, although the minimal dose associated with liver injury can range anywhere from 4 to 10 g. This dose will be substantially lower for younger adults.[28]
- Guaifenesin: The maximum recommended daily dose for Guaifenesin is 2.4 grams. Guaifenesin overdose up to 4 grams or more have not been associated with overdose symptoms, besides causing nausea and vomiting.[29] This should not be taken as a lead and guaifenesin overdose should be avoided if possible.
- Chlorphenamine: Known as an active ingredient in Coricidin, chlorphenamine acts as a blood pressure stabilizer. Chlorphenamine overdose has led to a number of circulatory complications and in combination with dextromethophan has resulted in many overdoses and death.[citation needed] Chlorphenamine should be avoided when taken in combination with DXM.
- Phenylephrine: The maximum daily dose should be under 60 milligrams. Phenylephrine overdose has been associated with a range of dangerous cardiovascular problems, which may be amplified further by dextromethorphan.[citation needed] Phenylephrine should be avoided when taken in combination with DXM.
- Pseudoephedrine: Overdose can cause cardiovascular problems.[citation needed] Pseudoephedrine should be avoided when taken in combination with DXM.
- Caffeine: Caffeine overdose can result from as few as 400 to 600 milligrams. May increase cardiovascular side effects of DXM, but the extent to this is unknown.[citation needed] Caffeine should be avoided when taken in combination with DXM.
- Diphenhydramine: Diphenhydramine in combination with dextromethorphan has a very low threshold for delirium and a complete detachment of reality. May also strain the circulatory system and heart in high doses.[citation needed]
- Aspirin: Aspirin overdose starts below 300mg/kg and can become deadly at 500mg/kg.[citation needed] Higher doses of aspirin should be avoided in combination with DXM.
- Dextromethorphan polistirex: This type of Dextromethorphan is used in the common brand Delsym for long cough and cold relive. The polistirex around the dextromethorphan causes the high of DXM to last much longer than intended, with some reports of the high lasting 18-24 hours instead of normal DXM lasting around 8-12 hours, which for some users could be very unpleasant. On top of that, you need more DXM polistirex to get the same high as regular DXM. This could lead to someone using too much DXM polistirex and overdosing to get the same effects as a moderate too high dose of normal DXM without the polistirex.
- Doxylamine: This drug is found in NyQuil and is used as a sleep aid so the user has an easier time sleeping. Doxylamine's median lethal dose (LD 50) is estimated to be 50–500 mg/kg in humans and when ingested with DXM will have a very low threshold for delirium and complete detachment of reality due to the fact that it is a generation one antihistamine just like Diphenhydramine. Doxylamine in combination with DXM can also cause strong sedation and ataxia when combined with each other which would lead to impaired thinking and possibly unconsciousness.
Potentiation
- Grapefruit juice:
Grapefruit juice is reported to be effective at potentiating the effects of DXM. For users who are drinking store bought syrup, this is useful as it means drinking less syrup. By inhibiting the enzyme CYP3A4, ingestion of grapefruit juice leads to DXM that would be metabolized into 3-MM, be metabolized into DXO instead. This leads to higher plasma concentrations of DXO, and slower degradation of DXO too, by inhibition of the conversion of DXO into 3-HM.
Near maximal potentiation remains for 4 hours after the drinking a cup (200–250 mL) of grapefruit juice, and drinking an amount beyond that doesn't increase effects significantly. On the other hand, frequent consumption of a cup at least three times a day, for a day or more, increases potentiation moreso than a lone (even if greater) quantity. After this, the potentiation also takes longer to wear off, suggesting frequent consumption of grapefruit juice has a cumulative effect.[30]
- Magnesium:
Magnesium presents synergy with virtually all dissociative substances due to magnesium's NMDAR antagonist properties. Dextromethorphan acts as a non-competitive antagonist at the NMDA receptor, binding to a site distinct from the ion channel pore where magnesium binds. Magnesium serves as a voltage-dependent blocker by occupying a site within the ion channel itself. This dual mechanism allows both substances to modulate the receptor’s activity in different ways, resulting in synergy.[31]
Preparation methods
Preparation methods for this compound within our tutorial index include:
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
The toxicity and long-term health effects of recreational DXM use in humans has not been studied in any scientific context and the exact toxic dosage is unknown.
Anecdotal evidence suggests that there do not seem to be any negative health effects attributed to simply trying DXM at low to moderate doses by itself and using it sparingly, however, many DXM users report that waiting a week between their respective plateaus before doing DXM again (one week after first plat., two weeks after 2nd plat., etc.) can potentially prevent or minimize severe damage to the kidneys and many other organs including the heart and liver due to an excess of assorted toxic chemicals in the blood stream after DXM use. This 1-4 week period gives your body time to filter out these chemicals and help get you back to baseline levels to where its safe to do again. Some more heavy users of DXM report that these chemicals can build up heavily over time if you do not follow these guidelines, which could mean waiting longer to do again after more heavy use between short periods. Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
Despite early speculation that DXM may cause neurotoxicity and Olney's lesions, it has not been shown to cause this effect in animals [32], However, many chronic users report significant issues with memory, attention, and mood that persist for many months after stopping usage. In rats,[33] oral administration of dextromethorphan did not cause neurotoxic effects in laboratory tests.[34] Oral administration of dextromethorphan repeatedly during adolescence, however, has been shown to impair learning in those rats during adulthood.[35]
It is strongly advised to use harm reduction practices if using this substance.
Dependence and abuse potential
As with other dissociatives, DXM produces dependence with chronic use and has moderate abuse potential. When dependence has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
A formal survey of dextromethorphan users[36] showed that more than half of users reported experience of the following withdrawal symptoms individually for the first week after long-term/addictive dextromethorphan use: fatigue, apathy, flashbacks, and constipation. Over a quarter reported insomnia, nightmares, inability to feel pleasure, impaired memory, attention deficit and decreased libido. Rarer side effects included panic attacks, impaired learning, tremor, yellowing of the skin, hives and muscle pain. Frequent and long-term usage at very high doses could lead to toxic psychosis and other permanent psychological problems.[3]
Tolerance to many of the effects of DXM develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. After that, it takes about 3 - 7 days for the tolerance to be reduced to half and 1 - 2 weeks to be back at baseline (in the absence of further consumption).
DXM produces cross-tolerance with all dissociatives, meaning that after the consumption of DXM all dissociatives will have a reduced effect.
Additionally, some users report an irreversible, permanent tolerance to DXM, which develops over a long period of time and is thought to correlate with the number of doses a person has ingested throughout their lifetime. Some users claim that there is a "50 trip limit", after which the rewarding and unique effects of DXM are said to disappear permanently. The reason for this is unknown, although it may be indicative of neurotoxicity.
Tolerance
Long-term dextromethorphan (DXM) abuse may result in a permanent irreversible tolerance due to several different mechanisms.
DXM is an NMDA receptor antagonist that blocks the inhibitory effects of glutamate in the brain. With repeated use, the body compensates for this blockade by increasing glutamate production. This leads to the rapid development of tolerance to DXM.[citation needed]
Chronic DXM abuse leads to neuroadaptations, including changes in neurons and synapses. These adaptive changes result in altered brain function that reduces the effects of DXM.[citation needed]
DXM exerts its effects by blocking NMDA receptors. Long-term abuse can lead to the downregulation of these receptors, meaning they are decreased in number. Reduced receptors result in diminished responsiveness to DXM.[citation needed]
DXM is metabolized in the liver. Chronic abuse can induce metabolic enzymes, which accelerate DXM breakdown.
Overdose
Anecdotal evidence suggests that the risk of DXM overdose becomes significant at roughly 15mg/kg to 20mg/kg, or roughly 1000mg - 1500mg in a 70kg person.[citation needed] DXM overdose can have a wide range of effects, including delusions, hallucinations, psychosis, confusion, panic attacks, mania, sedation and severe balance issues, sometimes very inappropriate or violent behavior, increased heart rate, nystagmus and amnesia.[citation needed]
More serious side effects include anesthesia, respiratory depression[citation needed], dangerously high fever, risk of accidental injury, self-harm and seizures. Seizures are created possibly due to hyponatremia and a general lowering of the seizure threshold.[37] They are reported above the 900-1000mg dose range[citation needed]. It is possible that DXM may also affect other levels like potassium, vitamins or blood sugar further contributing to seizures and other health problems in a linear fashion in terms of severity and duration.
It is also believed that extremely high and repeated doses lead to serotonin syndrome.[citation needed]
One should not disrupt a person undergoing this experience as their delusions may cause them to respond with violence. Care should be taken as to not let the user get injured, and medical attention, or at the very least, medical surveillance should be sought to prevent severe respiratory depression, choking or organ damage.
Death from DXM toxicity is rare, although most overdose cases do cite life-threatening complications, typically extremely elevated heart rate and blood pressure, urinary retention and rhabdomylosis. Fever and seizures can lead to brain cell death.[38]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Benzodiazepines - Small doses of benzodiazepines can end a bad trip, however, both substances potentiate the ataxia and sedation caused by the other. This can lead to unexpected loss of consciousness at high doses. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- Cannabis - CBD is known to be an inhibitor of the CYP2D6 enzyme, which normally breaks down DXM to Dextorphan. This can lead to unexpected effects.[39]
- DOx - The DOx class as psychedelic stimulants have the potential to mask the effects of DXM and could lead to redosing to an unsafe level. DXM can also potentiate DOx resulting in an unpleasantly intense experience. DOx are also very physically stimulating and can cause heart rate and blood pressure problems especially since both substances have a very long duration.
- 25x-NBOMe
- 2C-T-x - As most 2C-T-x's are MAOIs this can potentially lead to serotonin syndrome, among other dangerous effects.
- 5-MeO-xxT - Little information exists about this combination.
- Amphetamines - Both substances raise heart rate, in extreme cases, panic attacks caused by these drugs have led to more serious heart issues.
- Bupropion - Bupropion is a potent inhibitor of CYP2D6, the enzyme primarily responsible for breaking down DXM. This can lead to different and prolonged effects due to excessive accumulation of DXM in the bloodstream.[40] Recreationally mixing Bupropion with DXM can lower the seizure threshold, heighten anxiety/paranoia and increase the potential of tachycardia among other symptoms.[41] Information on this interaction within recreational doses is overall lesser-known and is to be advised against.
- Cocaine - Both substances raise heart rate, in extreme cases, panic attacks caused by these drugs have led to more serious heart issues.
- ΑMT
- PCP - Due to PCP's unique pharmacology affecting dopamine levels this may cause cardiovascular complications.
- MDMA - High risk of serotonin syndrome due to the fact that both MDMA and DXM are both serotonergic substances.
- Alcohol - Both substances potentiate the ataxia and sedation caused by the other and can lead to unexpected loss of consciousness at high doses. Place affected patients in the recovery position to prevent vomit aspiration from excess. Additionally CNS depression can lead to difficulty breathing. Avoid on anything higher than 1st plateau.
- GHB - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position. This combination is hard to predict
- GBL - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position. This combination is hard to predict
- Opioids - Both substances can, with heavy doses, cause respiratory depression, and additionally, extremely heavy doses of both may be hepatotoxic. Additionally, use of NMDA antagonists such as dextromethorphan can lower tolerance to and potentiate opioids, increasing their effects.
- Tramadol- Both substances can, with heavy doses, cause respiratory depression and if heavy doses are taken both may be hepatotoxic. Additionally, if one takes DXM, their tolerance of opiates goes down slightly, thus causing additional synergistic effects.
- MAOIs - High risk of serotonin syndrome.
- SSRIs - High risk of serotonin syndrome.
- Antihistamines - Many H1 antagonists, including antihistamines such Benadryl or terfenadine, as well as many antipsychotics, can slow down the metabolism of DXM due to their inhibition of CYP2D6, leading to possible death. [42]
Other interactions
- DXM has been shown to prevent and reverse morphine tolerance while also increasing analgesic effects[43][44][45] as well as potentiating the analgesic activity of NSAIDs, naproxen, piroxicam, etodolac, diclofenac, and ketorolac.[46]
Legal status
DXM is available either over the counter or by prescription in most countries. Some countries require the purchaser to be over 16, 18 or 21.
- Austria: Dextromethorphan is not listed in the "Suchtmittelgesetz" (Federal Law on Narcotics). Sales of DXM containing medications are restricted to pharmacies. DXM containing preparations are available at pharmacies without a prescription.[citation needed]
- Canada: Dextromethorphan is listed as specifically exempt from the Controlled Drugs and Substances Act.[47] It is available OTC in Canada and can legally be obtained in powder form.
- China: Dextromethorphan is a controlled substance(Psychotropic drug Class II"第二类精神药品") since July 1,2024[48].Sales of DXM containing medications are restricted to hospitals.However, The restriction is limited to the 'single drugs'. The combination of dextromethorphan and guaifenesin is still available as over-the-counter medicine now.
- Denmark: The only product containing dextromethorphan in Denmark, Dexofan brand tablets, is a prescription type A medication.[49]Template:Better source needed
- Finland: Dextromethorphan is listed in the medicinal product list and can only be sold in pharmacies.[50] It can be bought at any age and without a prescription.
- Germany: Dextromethorphan is not listed in the "Betäubungsmittelgesetz" (Federal Law on Narcotics).[51] Sales of DXM containing medications are restricted to pharmacies.[52] DXM containing preparations are available at pharmacies without a prescription.
- Mexico: Dextromethorphan is not listed in the General Health Law (Ley General de Salud),[53] which specifies which substances represent a risk to public health. It is also listed in the Reference Medicine Listing[54] as a General Health Law article 226 fraction VI drug, which means it can be freely sold even in businesses that are not legally registered as pharmacies. In practice, this translates into DXM-only syrups being available off-the-shelf and without prescription at any supermarket with a pharmacy section.
- Russia: Dextromethorphan is a schedule III controlled substance.[55]
- South Korea: Dextromethorphan is a controlled substance ("향정신성의약품 라목").However, 'combination drugs' contained DXM that meet the requirements are general drugs. If the single dose is 7.5 to 15 mg and the designated usage is within 60 mg per day, they are not controlled.[56]
- Sweden: Dextromethorphan is a controlled substance (narcotics class V)[57][58], and is no longer marketed in Sweden due to drug abuse and associated risks. The cough medicine Tussidyl, with DXM as active ingredient, was withdrawn from Swedish sales in 1999.[citation needed]
- Switzerland: Dextromethorphan is listed as a "Abgabekategorie B" pharmaceutical, which generally requires a prescription (it can also be given under advice from a pharmacist). Before being reclassified along other pharmaceutics in 2018, it was listed in category C, which meant it could be purchased over the counter.[59]
- Poland: Dextromethorphan is an over-the-counter drug, the purchaser must be over the age 18 and they cannot buy more than 360 mg of the substance at single pharmacy.[60]
- United States: Dextromethorphan is available over the counter and can be found in grocery stores, convenience stores, and online. Many physical locations requires the purchaser to be over the age of 18, however this is not the law.
See also
External links
Discussion
References
- ↑ Siu, A., Drachtman, R. (2007). "Dextromethorphan: a review of N-methyl-d-aspartate receptor antagonist in the management of pain". CNS drug reviews. 13 (1): 96–106. doi:10.1111/j.1527-3458.2007.00006.x. ISSN 1080-563X.
- ↑ Dicpinigaitis, P. V., Morice, A. H., Birring, S. S., McGarvey, L., Smith, J. A., Canning, B. J., Page, C. P. (April 2014). Sibley, D. R., ed. "Antitussive Drugs—Past, Present, and Future". Pharmacological Reviews. 66 (2): 468–512. doi:10.1124/pr.111.005116. ISSN 0031-6997.
- ↑ 3.0 3.1 3.2 3.3 Dextromethorphan (DXM), CESAR, 2018, retrieved 31 July 2018
- ↑ Yvette C. Terrie, Bsp. (1 November 2008). "Dextromethorphan Abuse". Pharmacy Times. 0.
- ↑ 5.0 5.1 5.2 5.3 Morris, H., Wallach, J. (August 2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–632. doi:10.1002/dta.1620. ISSN 1942-7611.
- ↑ "Memorandum for the Secretary of Defense" (PDF). Archived (PDF) from the original on 2017-09-15. Retrieved 2013-07-28.
- ↑ "Senate Bill No. 514" (PDF). An act to add Sections 11110 and 11111 to the Health and Safety Code, relating to nonprescription drugs. State of California, Legislative Counsel. Archived (PDF) from the original on 2018-03-08.
- ↑ Schwartz, A. R., Pizon, A. F., Brooks, D. E. (September 2008). "Dextromethorphan-induced serotonin syndrome". Clinical Toxicology (Philadelphia, Pa.). 46 (8): 771–773. doi:10.1080/15563650701668625. ISSN 1556-3650.
- ↑ 9.0 9.1 Hernandez, S. C., Bertolino, M., Xiao, Y., Pringle, K. E., Caruso, F. S., Kellar, K. J. (1 June 2000). "Dextromethorphan and Its Metabolite Dextrorphan Block α3β4 Neuronal Nicotinic Receptors ,". Journal of Pharmacology and Experimental Therapeutics. 293 (3): 962–967. ISSN 0022-3565.
- ↑ 10.0 10.1 Shin, E.-J., Nah, S.-Y., Chae, J. S., Bing, G., Shin, S. W., Yen, T. P. H., Baek, I.-H., Kim, W.-K., Maurice, T., Nabeshima, T., Kim, H.-C. (1 May 2007). "Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats". Neurochemistry International. 50 (6): 791–799. doi:10.1016/j.neuint.2007.01.008. ISSN 0197-0186.
- ↑ Werling, L. L., Keller, A., Frank, J. G., Nuwayhid, S. J. (October 2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder". Experimental Neurology. 207 (2): 248–257. doi:10.1016/j.expneurol.2007.06.013. ISSN 0014-4886.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 Reissig, C. J., Carter, L. P., Johnson, M. W., Mintzer, M. Z., Klinedinst, M. A., Griffiths, R. R. (September 2012). "High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens". Psychopharmacology. 223 (1): 1–15. doi:10.1007/s00213-012-2680-6. ISSN 0033-3158.
- ↑ 13.0 13.1 Pechnick, R. N., Poland, R. E. (1 May 2004). "Comparison of the Effects of Dextromethorphan, Dextrorphan, and Levorphanol on the Hypothalamo-Pituitary-Adrenal Axis". Journal of Pharmacology and Experimental Therapeutics. 309 (2): 515–522. doi:10.1124/jpet.103.060038. ISSN 0022-3565.
- ↑ 14.0 14.1 14.2 Nguyen, L., Thomas, K. L., Lucke-Wold, B. P., Cavendish, J. Z., Crowe, M. S., Matsumoto, R. R. (March 2016). "Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders". Pharmacology & Therapeutics. 159: 1–22. doi:10.1016/j.pharmthera.2016.01.016. ISSN 0163-7258.
- ↑ Jacqz-Aigrain, E., Cresteil, T. (1992). "Cytochrome P450-dependent metabolism of dextromethorphan: fetal and adult studies". Developmental Pharmacology and Therapeutics. 18 (3–4): 161–168. ISSN 0379-8305.
- ↑ 16.0 16.1 16.2 16.3 Yu, A., Haining, R. L. (November 2001). "Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?". Drug Metabolism and Disposition: The Biological Fate of Chemicals. 29 (11): 1514–1520. ISSN 0090-9556.
- ↑ Al-Jenoobi, F. I., Al-Thukair, A. A., Abbas, F. A., Ansari, M. J., Alkharfy, K. M., Al-Mohizea, A. M., Al-Suwayeh, S. A., Jamil, S. (January 2010). "Effect of black seed on dextromethorphan O- and N-demethylation in human liver microsomes and healthy human subjects". Drug Metabolism Letters. 4 (1): 51–55. doi:10.2174/187231210790980435. ISSN 1874-0758.
- ↑ Gorski, J. C., Jones, D. R., Wrighton, S. A., Hall, S. D. (5 July 1994). "Characterization of dextromethorphan N-demethylation by human liver microsomes. Contribution of the cytochrome P450 3A (CYP3A) subfamily". Biochemical Pharmacology. 48 (1): 173–182. doi:10.1016/0006-2952(94)90237-2. ISSN 0006-2952.
- ↑ Di Marco, M. P., Edwards, D. J., Wainer, I. W., Ducharme, M. P. (July 2002). "The effect of grapefruit juice and seville orange juice on the pharmacokinetics of dextromethorphan: The role of gut CYP3A and P-glycoprotein". Life Sciences. 71 (10): 1149–1160. doi:10.1016/S0024-3205(02)01799-X. ISSN 0024-3205.
- ↑ Zawertailo, L. A., Kaplan, H. L., Busto, U. E., Tyndale, R. F., Sellers, E. M. (August 1998). "Psychotropic Effects of Dextromethorphan Are Altered by the CYP2D6 Polymorphism: A Pilot Study". Journal of Clinical Psychopharmacology. 18 (4): 332–337. doi:10.1097/00004714-199808000-00014. ISSN 0271-0749.
- ↑ Kerry, N. L., Somogyi, A. A., Bochner, F., Mikus, G. (September 1994). "The role of CYP2D6 in primary and secondary oxidative metabolism of dextromethorphan: in vitro studies using human liver microsomes". British Journal of Clinical Pharmacology. 38 (3): 243–248. doi:10.1111/j.1365-2125.1994.tb04348.x. ISSN 0306-5251.
- ↑ Hou, C.-H., Tzeng, J.-I., Chen, Y.-W., Lin, C.-N., Lin, M.-T., Tu, C.-H., Wang, J.-J. (21 August 2006). "Dextromethorphan, 3-methoxymorphinan, and dextrorphan have local anaesthetic effect on sciatic nerve blockade in rats". European Journal of Pharmacology. 544 (1): 10–16. doi:10.1016/j.ejphar.2006.06.013. ISSN 0014-2999.
- ↑ Gorski, J. C., Jones, D. R., Wrighton, S. A., Hall, S. D. (July 1994). "Characterization of dextromethorphan N-demethylation by human liver microsomes". Biochemical Pharmacology. 48 (1): 173–182. doi:10.1016/0006-2952(94)90237-2. ISSN 0006-2952.
- ↑ New item… Zhang, W., Qin, L., Wang, T., Wei, S.-J., Gao, H.-M., Liu, J., Wilson, B., Liu, B., Zhang, W., Kim, H.-C., Hong, J.-S. (March 2005). "3‐Hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS‐induced neurotoxicity". The FASEB Journal. 19 (3): 1–25. doi:10.1096/fj.04-1586fje. ISSN 0892-6638.
- ↑ Shin, E.-J., Lee, P. H., Kim, H. J., Nabeshima, T., Kim, H.-C. (2008). "Neuropsychotoxicity of Abused Drugs: Potential of Dextromethorphan and Novel Neuroprotective Analogs of Dextromethorphan With Improved Safety Profiles in Terms of Abuse and Neuroprotective Effects". Journal of Pharmacological Sciences. 106 (1): 22–27. doi:10.1254/jphs.FM0070177. ISSN 1347-8613.
- ↑ Shin, E.-J., Bach, J.-H., Lee, S. Y., Kim, J. M., Lee, J., Hong, J.-S., Nabeshima, T., Kim, H.-C. (2011). "Neuropsychotoxic and Neuroprotective Potentials of Dextromethorphan and Its Analogs". Journal of Pharmacological Sciences. 116 (2): 137–148. doi:10.1254/jphs.11R02CR.
- ↑ Zawertalio, Laurie A. MSc, Kaplan, Howard L. PhD, Busto, Usoa E. PharmD, Tyndale, Rachel F. PhD, Sellers, Edward M. PhD (August 1998). "Psychotropic Effects of Dextromethorphan Are Altered by the CYP2D6 Polymorphism". Journal of Clinical Psychopharmacology. 18 (4): 332–337. doi:10.1097/00004714-199808000-00014.
- ↑ Dimitropoulos, E., Ambizas, E. M. (2014), Acetaminophen Toxicity: What Pharmacists Need to Know, U.S. Pharmacist
- ↑ https://www.medsafe.govt.nz/Consumers/cmi/CoughandCold/Guaifenesin1.pdf
- ↑ Bailey, D. G., Dresser, G., Arnold, J. M. O. (5 March 2013). "Grapefruit–medication interactions: Forbidden fruit or avoidable consequences?". CMAJ. 185 (4): 309–316. doi:10.1503/cmaj.120951. ISSN 0820-3946.
- ↑ Chiavetta, L. (2021). Coadministration of ketamine and magnesium: An integrative review.
- ↑ Erowid DXM Vaults : Health : The Bad News Isn’t In : A Look at Dissociative-Induced Brain Damage, by Anderson C
- ↑ Hashimoto, K., Tomitaka, S., Narita, N., Minabe, Y., Iyo, M., Fukui, S. (July 1996). "Induction of heat shock protein HSP-70 in rat retrosplenial cortex following administration of dextromethorphan". Environmental Toxicology and Pharmacology. 1 (4): 235–239. doi:10.1016/1382-6689(96)00016-6. ISSN 1382-6689.
- ↑ Carliss, R. D., Radovsky, A., Chengelis, C. P., O’Neill, T. P., Shuey, D. L. (July 2007). "Oral administration of dextromethorphan does not produce neuronal vacuolation in the rat brain". Neurotoxicology. 28 (4): 813–818. doi:10.1016/j.neuro.2007.03.009. ISSN 0161-813X.
- ↑ Recreational use of dextromethorphan, 2022
- ↑ Ziaee, V., Akbari Hamed, E., Hoshmand, A., Amini, H., Kebriaeizadeh, A., Saman, K. (September 2005). "Side effects of dextromethorphan abuse, a case series". Addictive Behaviors. 30 (8): 1607–1613. doi:10.1016/j.addbeh.2005.02.005. ISSN 0306-4603.
- ↑ DXM & CPM - Erowid Exp - “Death from 28 Coriciden Pills”
- ↑ DXM - Erowid Exp - “DXM Overdose”
- ↑ Yamaori, S., Okamoto, Y., Yamamoto, I., Watanabe, K. (November 2011). "Cannabidiol, a Major Phytocannabinoid, As a Potent Atypical Inhibitor for CYP2D6". Drug Metabolism and Disposition. 39 (11): 2049–2056. doi:10.1124/dmd.111.041384. ISSN 0090-9556.
- ↑ Kotlyar, Michael; Brauer, Lisa H; Tracy, Timothy S; Hatsukami, Dorothy K; Harris, Jennifer; Bronars, Carrie A; Adson, David E (10 February 2005). "Inhibition of CYP2D6 activity by bupropion". Journal of Clinical Psychopharmacology. 10. doi:10.1097/01.jcp.0000162805.46453.e3. ISSN 0271-0749.
- ↑ Huecker, Martin R.; Smiley, Abbey; Saadabadi, Abdolreza (9 April 2023), Bupropion
- ↑ Kintz, P., Mangin, P. (December 1992). "Toxicological findings in a death involving dextromethorphan and terfenadine". The American Journal of Forensic Medicine and Pathology. 13 (4): 351–352. doi:10.1097/00000433-199212000-00018. ISSN 0195-7910.
- ↑ Elliott, K., Hynansky, A., Inturrisi, C. E. (December 1994). "Dextromethorphan attenuates and reverses analgesic tolerance to morphine". Pain. 59 (3): 361–368. doi:10.1016/0304-3959(94)90022-1. ISSN 0304-3959.
- ↑ Mao, J., Price, D. D., Caruso, F. S., Mayer, D. J. (October 1996). "Oral administration of dextromethorphan prevents the development of morphine tolerance and dependence in rats". Pain. 67 (2–3): 361–368. doi:10.1016/0304-3959(96)03120-x. ISSN 0304-3959.
- ↑ Asl, B. H., Hassanzadeh, K., Khezri, E., Mohammadi, S. (1 July 2008). "Evaluation the effects of dextromethorphan and midazolam on morphine induced tolerance and dependence in mice". Pakistan journal of biological sciences: PJBS. 11 (13): 1690–1695. doi:10.3923/pjbs.2008.1690.1695. ISSN 1028-8880.
- ↑ Price, D. D., Mao, J., Lu, J., Caruso, F. S., Frenk, H., Mayer, D. J. (November 1996). "Effects of the combined oral administration of NSAIDs and dextromethorphan on behavioral symptoms indicative of arthritic pain in rats". Pain. 68 (1): 119–127. ISSN 0304-3959.
- ↑ Controlled Drugs and Substances Act (S.C. 1996, c. 19) | http://laws-lois.justice.gc.ca/PDF/C-38.8.pdf
- ↑ [1]
- ↑ "Availability and price table of Dexofan at medicin.dk" (in Danish).
- ↑ Fimea - Lääkealan turvallisuus- ja kehittämiskeskuksen päätös lääkeluettelosta
- ↑ BtMG - nichtamtliches Inhaltsverzeichnis
- ↑ AMG - nichtamtliches Inhaltsverzeichnis
- ↑ https://web.archive.org/web/20171117202316/http://www.diputados.gob.mx/LeyesBiblio/pdf/142_220617.pdf
- ↑ https://www.gob.mx/cms/uploads/attachment/file/178695/LMR_2017-07_V001.pdf
- ↑ Постановление Правительства РФ от 30.06.1998 N 681 “Об утверждении перечня наркотических средств, психотропных веществ и их прекурсоров, подлежащих контролю в Российской Федерации” (с изменениями и дополнениями), ГАРАНТ
- ↑ https://en.namu.wiki/w/%EB%8D%B1%EC%8A%A4%ED%8A%B8%EB%A1%9C%EB%A9%94%ED%86%A0%EB%A5%B4%ED%8C%90
- ↑ Regeringskansliets rättsdatabaser
- ↑ With the exception of preparations for medical or scientific use in the form of solutions, not containing more than 3 mg/ml.
- ↑ List of intended reallocations of medicinal products for human use in dispensing category C, swissmedic, 2018
- ↑ The law against drug use, Polish Sejm, 2016