NBx
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |
N-Benzylphenethylamine (also known as NBx) is a general classification of phenethylamines which have a benzyl substitution on the nitrogen atom (N-benzyl substitution).
The most recognized members include the psychedelic 25x-NBOMe and 25x-NBOH series of psychedelics. They have often been misrepresented as LSD, which has lead to numerous accidental overdoses, difficult experiences, health problems, and fatalities.
There are also unsubstituted or simple N-Benzylphenethylamines, but they are typically not significantly active and lesser known.
It is strongly recommended that one should inform themselves first and most importantly with these. Both of them (25x-NBOx) are the only compounds which are largely distributed as well are associated with major risks, dangers and fatalities, which are not found for many of the other NBx compounds listed here.
Chemistry
In a comparison of NBx and phenethylamine chemicals, each NBx molecule is a N-benzyl derivative of the corresponding phenethylamine molecule. This change in structure generally results a minor or major increase in potency, depending on the substituents on both the phenethylamine and benzyl parts.
The term NBx denotes the addition from a phenethylamine backbone through a substitution at the amine (NH2) with a benzyl (B) group with any substitutions on it(x).
There are many possible N-benzylphenethylamine compounds which can be further devided into several sub-classes. It should be noted that most of these sub-categories have different nomenclatures for their naming rules.
x denotes possible substituent as a character relevant to the sub-class.
… denotes any or no substituent as a characted not relevant to the sub-class.
| X-NB… This subcategory is focused on the phenyl ring of the phenethylamine part of the molecule. |
|---|
|
25x-NB… - 25x-NB… compounds are derived from the 2C-x series, where 25 refers to the two methoxy substitutions on positions 2 and 5 position on the phenyl ring and x denotes any substitution by the letter/-s x from the corresponding 2C-x. x1x2-NB… - If the phenethylamine part of the N-benzylderivative does not have an official recognized name, but is substituted with only methoxy groups at positions 2, 3, 5 and/or 6, then x1 will be designated as the position/-s of the methoxy groups attached to the phenyl ring. x2 denotes the substitution at position 4.[1]
x-NB… or NB…-x - Where x can denote a named phenethylamine compound. They should be regarded as obsolete if other notations can be used instead. (e.g. 25i-NBOMe should be used instead of 2C-I-NBOMe).
|
| …NBX This subcategory is focused on the phenyl ring of the N-benzyl substitution of the molecule. |
|---|
|
…NB - Simple N-benzyl substitution.
…NBx1x2OMe - OMe denotes a methoxy group. If a methoxy group is fused at positions other than 2, its position is designated at x1. x2 designates a possibble prefix if multiple substituents are present.
…NBx1x2OH - OH denotes a hydoxy group. If a methoxy group is fused at positions other than 2, its position is designated at x1. x2 designates a possibble prefix if multiple substituents are present.
…NBx1x2X - X denotes any halogen substituent (F, Cl, Br, I). If a methoxy group is fused at positions other than 2, its position is designated at x1. x2 designates a possibble prefix if multiple substituents are present.
…NBxMD - MD denotes a fused methlenedioxy ring. If MD is fused at positions other than 2 and 3, its positions are designated at x1.
|
| IUPAC names Partially or fully not correlated to NBx nomenclature |
|---|
|
Examples: MDBZ, Clobenzorex, C30-NBOMe, DMBMPP, FECIMBI-36. |
| Others |
|---|
|
Examples: 25I-NMeTh, 2C-B-AN, 25B-NAcPip, 5MT-NBOMe 25B-NMe7DHBF, 25I-NMe7DHBF, 25I-N2Nap1OH, 25I-N4MT3M . |
List of N-benzylphenethylamine compounds
| Compound | R | Cyc | Structure |
|---|---|---|---|
| 25B-NB | 2,5-dimethoxy-4-bromo | phenyl | 125px |
| 25C-NB | 2,5-dimethoxy-4-chloro | phenyl | 125px |
| 25I-NB | 2,5-dimethoxy-4-iodo | phenyl | 125px |
| 25I-NMeTh | 2,5-dimethoxy-4-iodo | thiophen-2-yl | 125px |
| 25B-NMePyr | 2,5-dimethoxy-4-bromo | pyridin-2-yl | 125px |
| 25I-NMeFur | 2,5-dimethoxy-4-iodo | furan-2-yl | 125px |
| 25I-NMeTHF | 2,5-dimethoxy-4-iodo | tetrahydrofuran-2-yl | 125px |
| 25B-NBOH | 2,5-dimethoxy-4-bromo | 2-hydroxyphenyl | 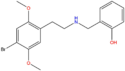
|
| 25B-NBOMe | 2,5-dimethoxy-4-bromo | 2-methoxyphenyl | 125px |
| 25B-NB23DM | 2,5-dimethoxy-4-bromo | 2,3-dimethoxyphenyl | 125px |
| 25B-NB25DM | 2,5-dimethoxy-4-bromo | 2,5-dimethoxyphenyl | 125px |
| 25B-NMe7BF | 2,5-dimethoxy-4-bromo | benzofuran-7-yl | 125px |
| 25B-NMe7DHBF | 2,5-dimethoxy-4-bromo | 2,3-dihydrobenzofuran-7-yl | 125px |
| 25B-NMe7BT | 2,5-dimethoxy-4-bromo | benzothiophen-7-yl | 125px |
| 25B-NMe7Box | 2,5-dimethoxy-4-bromo | benzoxazol-7-yl | 125px |
| 25B-NMe7Ind | 2,5-dimethoxy-4-bromo | indol-7-yl | 125px |
| 25B-NMe7Indz | 2,5-dimethoxy-4-bromo | indazol-7-yl | 125px |
| 25B-NMe7Bim | 2,5-dimethoxy-4-bromo | benzimidazol-7-yl | 125px |
| FECIMBI-36 | 2,5-dimethoxy-4-bromo | 2-(2-fluoroethoxy)phenyl | 125px |
| DOB-NBOMe | 2,5-dimethoxy-4-bromo | 2-methoxyphenyl | 125px |
| 25C-NB3OMe | 2,5-dimethoxy-4-chloro | 3-methoxyphenyl | 
|
| 25C-NB4OMe | 2,5-dimethoxy-4-chloro | 4-methoxyphenyl | 
|
| C30-NBOMe | 2,5-dimethoxy-4-chloro | 3,4,5-trimethoxyphenyl | 125px |
| 25C-NBF | 2,5-dimethoxy-4-chloro | 2-fluorophenyl | 
|
| 25C-NBCl | 2,5-dimethoxy-4-chloro | 2-chlorophenyl | 125px |
| 25C-NBOH | 2,5-dimethoxy-4-chloro | 2-hydroxyphenyl | 125px |
| 25C-NBOMe | 2,5-dimethoxy-4-chloro | 2-methoxyphenyl | 
|
| 25C-NBOEt | 2,5-dimethoxy-4-chloro | 2-ethoxyphenyl | 125px |
| 25C-NBOiPr | 2,5-dimethoxy-4-chloro | 2-isopropoxyphenyl | 125px |
| 25F-NBOMe | 2,5-dimethoxy-4-fluoro | 2-methoxyphenyl | 125px |
| 25CN-NBOH | 2,5-dimethoxy-4-cyano | 2-hydroxyphenyl | 125px |
| 25CN-NBOMe | 2,5-dimethoxy-4-cyano | 2-methoxyphenyl | 
|
| 25D-NBOMe | 2,5-dimethoxy-4-methyl | 2-methoxyphenyl | 
|
| 25D-NBOH | 2,5-dimethoxy-4-methyl | 2-hydroxyphenyl | 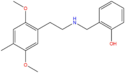
|
| 25E-NBOMe | 2,5-dimethoxy-4-ethyl | 2-methoxyphenyl | 
|
| 25E-NBOH | 2,5-dimethoxy-4-ethyl | 2-hydroxyphenyl | 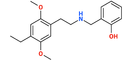
|
| 25G-NBOMe | 2,5-dimethoxy-3,4-dimethyl | 2-methoxyphenyl | 
|
| 25H-NBOMe | 2,5-dimethoxy | 2-methoxyphenyl | 
|
| 25I-NB34MD | 2,5-dimethoxy-4-iodo | 3,4-methylenedioxyphenyl | 
|
| 25I-NB3OMe | 2,5-dimethoxy-4-iodo | 3-methoxyphenyl | 
|
| 25I-NB4OMe | 2,5-dimethoxy-4-iodo | 4-methoxyphenyl | 
|
| 25I-NBF | 2,5-dimethoxy-4-iodo | 2-fluorophenyl | 
|
| 25I-NBBr | 2,5-dimethoxy-4-iodo | 2-bromophenyl | 125px |
| 25I-NBTFM | 2,5-dimethoxy-4-iodo | 2-(trifluoromethyl)phenyl | 125px |
| 25I-NBMD | 2,5-dimethoxy-4-iodo | 2,3-methylenedioxyphenyl | 
|
| 25B-NBMD | 2,5-dimethoxy-4-bromo | 2,3-methylenedioxyphenyl | 125px |
| 25C-NBMD | 2,5-dimethoxy-4-chloro | 2,3-methylenedioxyphenyl | 125px |
| 25D-NBMD | 2,5-dimethoxy-4-methyl | 2,3-methylenedioxyphenyl | 125px |
| 25I-NBOH | 2,5-dimethoxy-4-iodo | 2-hydroxyphenyl | 
|
| 25I-NBOMe | 2,5-dimethoxy-4-iodo | 2-methoxyphenyl | 
|
| DOI-NBOMe | 2,5-dimethoxy-4-iodo | 2-methoxyphenyl | 125px |
| 25I-NBMeOH | 2,5-dimethoxy-4-iodo | 2-(hydroxymethyl)phenyl | 125px |
| 25I-NBAm | 2,5-dimethoxy-4-iodo | 2-(carbamoyl)phenyl | 125px |
| 25I-NMe7DHBF | 2,5-dimethoxy-4-iodo | 2,3-dihydrobenzofuran-7-yl | 125px |
| 25I-N2Nap1OH | 2,5-dimethoxy-4-iodo | 1-hydroxynaphthalen-2-yl | 125px |
| 25I-N3MT2M | 2,5-dimethoxy-4-iodo | 3-methoxythiophen-2-yl | 125px |
| 25I-N4MT3M | 2,5-dimethoxy-4-iodo | 4-methoxythiophen-3-yl | 125px |
| 25iP-NBOMe | 2,5-dimethoxy-4-isopropyl | 2-methoxyphenyl | |
| 25N-NBOMe | 2,5-dimethoxy-4-nitro | 2-methoxyphenyl | 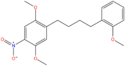
|
| 25P-NBOMe | 2,5-dimethoxy-4-propyl | 2-methoxyphenyl | 
|
| 25P-NBOH | 2,5-dimethoxy-4-propyl | 2-hydroxyphenyl | 125px |
| 25TFM-NBOMe | 2,5-dimethoxy-4-(trifluoromethyl) | 2-methoxyphenyl | 
|
| 25T-NBOMe | 2,5-dimethoxy-4-(methylthio) | 2-methoxyphenyl | 125px |
| 25T2-NBOMe | 2,5-dimethoxy-4-(ethylthio) | 2-methoxyphenyl | 125px |
| 25T4-NBOMe | 2,5-dimethoxy-4-(isopropylthio) | 2-methoxyphenyl | 125px |
| 25T7-NBOMe | 2,5-dimethoxy-4-(propylthio) | 2-methoxyphenyl | 125px |
| 25T7-NBOH | 2,5-dimethoxy-4-(propylthio) | 2-hydroxyphenyl | 125px |
| NBOMe-mescaline | 3,4,5-trimethoxy | 2-methoxyphenyl | 
|
| NBOMe-escaline | 3,5-dimethoxy-4-ethoxy | 2-methoxyphenyl | 125px |
| MDPEA-NBOMe | 3,4-methylenedioxy | 2-methoxyphenyl | 125px |
| MDBZ | 3,4-methylenedioxy | phenyl | 
|
| Clobenzorex | H | 2-chlorophenyl | |
| 4-EA-NBOMe | 4-ethyl | 2-methoxyphenyl | 125px |
| 5-APB-NBOMe | benzofuran-5-yl instead of phenyl | 2-methoxyphenyl | 125px |
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
Dependence and abuse potential
Members of the psychedelic-acting sub-series of N-benzylphenethylamines are not habit-forming and the desire to use them can actually decrease with use. They are thought to be most often self-regulating.
Some N-benzylphenethylderivatives have primarily stimulating effects which can carry risk of being habit-forming, although most of these compounds are not very active compared to traditional stimulants which have a decreased risk of being addicting.
This can also be applied to tolerance:
Tolerance to most if not all members of the psychedelic derivatives are built almost immediately after ingestion. After that, it takes about 1 week for the tolerance to be reduced to half and 2 weeks to be back to baseline (in the absence of further consumption). Members of the 25x-NBOMe series present cross-tolerance with all psychedelics, meaning that after the consumption of any member of the 25x-NBOMe series all psychedelics will have a reduced effect.
Tolerance of most members of the stimulating derivatives are built after consecutive dosing for several days. After that, it takes about 1 week for the tolerance to be reduced to half and 2 weeks to be back to baseline (in the absence of further consumption). Members of the stimulating derivatived present cross-tolerance with all stimulants, meaning that after the consumption of any member of these derivatives, stimulants will have a reduced effect.
Overdose
Overdoses are serious regarding especially the 25x-NBOMe series an to a lesser extent the 25x-NBOH series. Due to the very high potency and seemingly unpredictable effects the margin between a normal and an overdose of NBOMe compounds is extremely small when compared to many other substances. The exact toxic dose is unclear since it seems to depend a lot on personal physiology, rather than predominantly dose. However, various anecdotal reports suggest that dangerous side effects begin to appear when exceeding 1000 μg and it possibly becoming lethal for the more sensitive people at roughly 2000 μg. Reports of other people surviving much higher doses, sometimes even without any major side effects have been documented as well.
There is also the uncertainty of dosage on blotter paper since it is rather difficult to lay such an exact dosage. Insufflating, vaporizing or drinking tinctures of this substance is highly discouraged because of this and has been tied to many documented deaths[2][3][4]. One study found that 25I‐NBOMe and 25C‐NBOMe blotter papers contained 'hotspots' with higher quantities of the drug, implying an inherent risk of overdosing.[5]
The overdose effects of NBOMes are typically a dangerously high heart rate, blood pressure, hyperthermia and significant vasoconstriction[6][7] also accompanied by confusion, delusions, panic attacks, aggressive behavior, numbness or pain, amnesia and often seizures. The risks in an overdose include anything from organ failure to cardiac arrest and death[citation needed]. There are also multiple reports of people lethally injuring themselves or falling to death[8][9]. Benzodiazepines or antipsychotics can help with the psychological effects during an overdose although medical attention should always be called in even a possible overdose of 25I-NBOMe.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit. Due to the highly unpredictable nature of the NBOMe series, it is generally advised to avoid mixing them with other psychoactive substances.
- 2C-T-X - The 2C-T-X phenethylamines can be unpredictable in their interactions and the NBOMes are known to be unpredictable even alone. As a result, this combination should be avoided.
- 5-MeO-xxt - The 5-MeO tryptamines can be unpredictable in their interactions and the NBOMes are known to be unpredictable even alone. As a result, this combination should be avoided.
- Amphetamines - Amphetamines and NBOMes both provide considerable stimulation. When combined they can result in tachycardia, hypertension, vasoconstriction and, in extreme cases, heart failure. The anxiogenic and focusing effects of stimulants are also not good in combination with psychedelics as they can lead to unpleasant thought loops. NBOMes are known to cause seizures and stimulants can increase this risk.
- aMT
- Caffeine - Caffeine can bring out the natural stimulation from psychedelic drugs to make it uncomfortable. High doses can cause anxiety which is hard to handle while tripping.
- Cannabis - Cannabis has an unexpectedly strong and unpredictable synergy with the effects of psychedelics. Caution is advised with this combination as it can significantly increase the risk of adverse psychological reactions like anxiety, paranoia, panic attacks, and psychosis. Users are advised to start off with only a fraction of their normal cannabis dose and take long breaks between hits to avoid over intake.
- Cocaine - Cocaine and NBOMes both provide considerable stimulation. When combined they can result in severe vasoconstriction, tachycardia, hypertension, and in extreme cases heart failure.
- DOx
- DXM
- Lithium - Lithium is commonly prescribed in the treatment of [https://en.wikipedia.org/wiki/Bipolar_disorder bipolar disorder. There is a large body of anecdotal evidence that suggests taking it with psychedelics significantly increases the risk of psychosis and seizures. As a result, this combination is strictly discouraged.
- MAOIs - MAO-B inhibitors can increase the potency and duration of phenethylamines unpredictably.
- MDMA
- MXE - As an NMDA antagonist, MXE potentiates NBOMes which can be unpleasantly intense.
- Tramadol - Tramadol is well known to lower seizure threshold and NBOMes have also shown a tendency to cause severe seizures
Serotonin syndrome risk
Combinations with the following substances can cause dangerously high serotonin levels. Serotonin syndrome requires immediate medical attention and can be fatal if left untreated.
- MAOIs - Such as banisteriopsis caapi, syrian rue, phenelzine, selegiline, and moclobemide.[10]
- Serotonin releasers - Such as MDMA, 4-FA, methamphetamine, methylone and αMT.
- SSRIs - Such as citalopram and sertraline
- SNRIs - Such as tramadol and venlafaxine
- 5-HTP
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
- United States: In the US, some of the NBx chemicals are listed as Schedule I substances and others may be considered analogues under the Federal Analogue Act.[citation needed]
- United Kingdom: The majority of synthesised NBx substances are Class A drugs in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause.[11] Any compounds not covered by the clause are illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26th, 2016.[12]
See also
External links
Literature
- Eline Pottie1, Olga V. Kupriyanova2,3, Asher L. Brandt4, Robert B. Laprairie4,5, Vadim A. Shevyrin6, Christophe P. Stove1 (2021). Serotonin 2A receptor (5-HT2AR) activation by 25H-NBOMe positional isomers: in vitro functional evaluation and molecular docking. https://doi.org/10.1021/acsptsci.0c00189
- Amy J Eshleman, Katherine M Wolfrum, John F Reed, Sunyoung O Kim, Robert A Johnson, Aaron Janowsky (2018). Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: high potency agonists at 5-HT2A receptors. https://doi.org/10.1016/j.bcp.2018.09.024
- Hansen, M., Phonekeo, K., Paine, J. S., Leth-Petersen, S., Begtrup, M., Bräuner-Osborne, H., & Kristensen, J. L. (2014). Synthesis and structure–activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chemical Neuroscience, 5(3), 243-249. https://doi.org/10.1021/cn400216u
- Heim, Ralf (2004). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur". Freie Universität Berlin. Retrieved 27 June 2015.
- Hansen, M.; Phonekeo, K.; Paine, J. S.; Leth-Petersen, S.; Begtrup, M.; Bräuner-Osborne, H.; Kristensen, J. L. (2014). "Synthesis and Structure-Activity Relationships of N-Benzyl Phenethylamines as 5-HT2A/2C Agonists". ACS Chemical Neuroscience. 5 (3): 243–9. PMC 3963123 Freely accessible. PMID 24397362. https://doi.org/10.1021/cn400216u
- Ettrup, A.; Hansen, M.; Santini, M. A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M. M.; Madsen, J. (2010). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–693. PMID 21174090. https://doi.org/10.1007/s00259-010-1686-8
References
- ↑ Pottie, E., Kupriyanova, O. V., Brandt, A. L., Laprairie, R. B., Shevyrin, V. A., Stove, C. P. (9 April 2021). "Serotonin 2A Receptor (5-HT 2A R) Activation by 25H-NBOMe Positional Isomers: In Vitro Functional Evaluation and Molecular Docking". ACS Pharmacology & Translational Science. 4 (2): 479–487. doi:10.1021/acsptsci.0c00189. ISSN 2575-9108.
- ↑ Erowid 25I-NBOMe (2C-I-NBOMe) Vault : Fatalities / Deaths
- ↑ Erowid 2C-C-NBOMe (25C-NBOMe) Vault : Fatalities / Deaths
- ↑ Erowid NBOMe (Other or Unknown NBOMe-Compound) Vault : Fatalities / Deaths
- ↑ Lützen, E., Holtkamp, M., Stamme, I., Schmid, R., Sperling, M., Pütz, M., Karst, U. (April 2020). "Multimodal imaging of hallucinogens 25C‐ and 25I‐NBOMe on blotter papers". Drug Testing and Analysis. 12 (4): 465–471. doi:10.1002/dta.2751. ISSN 1942-7603.
- ↑ Marchi, N. C., Scherer, J. N., Fara, L. S., Remy, L., Ornel, R., Reis, M., Zamboni, A., Paim, M., Fiorentin, T. R., Wayhs, C. A. Y., Von Diemen, L., Pechansky, F., Kessler, F. H. P., Limberger, R. P. (1 March 2019). "Clinical and Toxicological Profile of NBOMes: A Systematic Review". Psychosomatics. 60 (2): 129–138. doi:10.1016/j.psym.2018.11.002. ISSN 0033-3182.
- ↑ Yoon, K. S., Yun, J., Kim, Y.-H., Shin, J., Kim, S. J., Seo, J.-W., Hyun, S.-A., Suh, S. K., Cha, H. J. (1 April 2019). "2-(2,5-Dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe) and N-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25C-NBOMe) induce adverse cardiac effects in vitro and in vivo". Toxicology Letters. 304: 50–57. doi:10.1016/j.toxlet.2019.01.004. ISSN 0378-4274.
- ↑ https://psychonautwiki.org/wiki/File:Nbome_death_news_i2013e0190_disp.jpg
- ↑ https://psychonautwiki.org/wiki/File:Nbome_death_news_i2013e0191_disp.jpg
- ↑ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210
 . eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- ↑ United Kingdom. (2014). Misuse of Drugs Act 1971 (S.I. 2014/1106). London: The Stationery Office Limited. Retrieved July 5, 2017, from http://www.legislation.gov.uk/uksi/2014/1106/made
- ↑ Psychoactive Substances Act 2016 (Legislation.gov.uk) | http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted
