GBL

Fatal overdose may occur when GABAergic substances are combined with other depressants such as opiates, benzodiazepines, barbiturates, gabapentinoids, thienodiazepines or alcohol.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: GBL |
| GBL | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | GBL, gamma-Butyrolactone | ||||||||||||||||||||||||||||||||
| Substitutive name | 1,4-lactone, 4-butyrolactone, 4-hydroxybutyric acid lactone, gamma-hydroxybutyric acid lactone, and oxolan-2-one | ||||||||||||||||||||||||||||||||
| Systematic name | Dihydrofuran-2(3H)-one | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||||
| Chemical class | Lactone | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
| Nitrous | |||||||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||
| Benzodiazepines | |||||||||||||||||||||||||||||||||
gamma-Butyrolactone (also known as γ-butyrolactone and commonly as GBL) is a synthetic depressant substance of the lactone chemical class that produces powerful euphoric and disinhibiting effects similar to that of alcohol intoxication. In humans it acts as a prodrug for GHB, where 1ml is equivalent to 1.66 grams of the sodium salt of GHB (Sodium oxybate).[citation needed]
GBL is a common solvent and reagent in chemistry and is also used as a flavoring, stain remover, wheel cleaner, superglue remover, paint stripper, and as a solvent in some wet aluminum electrolytic capacitors.[citation needed] It has also been found in some wine products, which suggests it may be a naturally occurring substance (see section below).
GBL, along with 1,4-butanediol, are known to dissolve most types of plastic over time.[2] For this reason, it is recommended to only transport and store the drug using a glass container, standard gelatin capsules (not vegetarian), or high-density polyethylene plastic (also known as #2 recyclable plastic). To check the type of plastic used on a bottle, one can look at the bottom for a number in the triangle shaped recycling label. GBL reacts and breaks down various forms of plastic (including plastic lids) within seconds and will float to the bottom of a container while dissolved plastic moves up. It is currently unknown how far plastic can be broken down, but there may be serious consequences when ingesting microplastics. To avoid this, do not handle GBL at any stage with ordinary plastic, for example with plastic syringes or glass bottles with an ordinary plastic cap. The liquid should be perfectly clear and colorless.
GBL has reportedly been used as date rape drug (as a legal alternative to GHB), in which it is secretly put dropwise into drinks but there is little evidence to support it being widespread, especially compared to alcohol due to its strong taste. GBL and GHB are also referred to as "K.-o.-Tropfen" (K.-o.-drops) in German-speaking countries. Care should be taken when offered drinks from strangers.
Chemistry
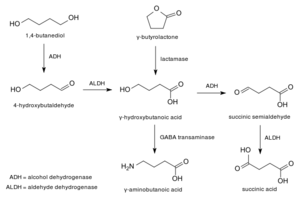
GBL (gamma-butyrolactone) is a cyclic ester of gamma-hydroxybutanoic acid. GBL can be synthesized from GHB through dehydration of the terminal hydroxy groups, forming a saturated lactone ring. Structurally, GBL is a five-membered ring with an oxygen substituent at R1, adjacent from the oxygen atom in the ring, forming a cyclic ester called a lactone.
Physically, it is a hygroscopic colorless oily liquid with a weak characteristic odor and is soluble in water.
GBL has been found in extracts from samples of unadulterated wines.[3] This finding indicates that GBL is a naturally occurring component in some wines and may be present in similar products. The concentration detected was approximately 5 μg/mL and was easily observed using a simple extraction technique followed by GC/MS analysis.
It can also be found in cheese flavorings but typically results in a content of 0.0002% GBL in the final foodstuff.[4]
Due to its chemical similarity, GBL can easily be converted into GHB by calendistine laboratories in a one-step synthesis.[citation needed]
Pharmacology
GBL is presumably not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB[5]. Hoewever, GBL may display other effects on the body, unlike GHB, but it is currently not known. It is theorized to induce a state of metabolic acidosis, a significant medical condition, especially when used for longer periods of time.[6]
It is rapidly converted into GHB by lactonase enzymes found in the blood.[5] GBL is more lipophilic (fat soluble) than GHB, and so is absorbed faster and has higher bioavailability; paradoxically this can mean that GBL has a faster onset of effects than GHB itself, even though it is a prodrug.
The levels of lactonase enzyme can vary between individuals, meaning that first-time users can show unpredictable results, even from small doses. In many this manifests as slow onset of effects, followed by headaches, semi-consciousness which is distinct from GBL sleep in normal users. If the user decides to try again at a later date, they appear to be able to enjoy the effects normally. Because of these pharmacokinetic differences, GBL tends to be more potent and faster-acting than GHB, but has a shorter duration; whereas the related compound 1,4-butanediol (1,4-B) tends to be slightly less potent, slower to take effect but longer-acting than GHB.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Reportedly, GBL is very similar to GHB. More muscle relaxing and less prone to give clonic muscle movements than GHB. Slightly longer acting, and has a more sedative feeling than GHB. May easily give gastrointestinal disturbances like nausea, diarrhoea and gas. Comment: It is not clear if ingesting butyrolactone is toxic or not. It is not cancerogenic as some sources say, but is probably not good for you. The taste is extremely disagreeable.[7]
Physical effects 
-
- Stimulation & Sedation - At lower dosages, GBL is physically stimulating, encouraging movement and wakefulness. At higher dosages, however, it becomes physically sedating, encouraging sleep and lethargy.
- Respiratory depression - In cases of GBL overdoses, many report experiencing an abnormal pattern of breathing characterized by progressively deeper and sometimes faster breathing, followed by a gradual decrease that results in a temporary stop in breathing called an apnea.
- Muscle relaxation - Muscle relaxation is reportedly more prevalent with GBL than GHB and it is less prone to causing convulsions.
- Tactile suppression & Tactile enhancement - Both numbing as well as enhancing tactile effects can be felt coincidingly.
- Nausea - This effect is more common than with GHB and may persist when GBL is used frequently.
- Stomach pain - Stomach discomfort or pain is more common with GBL than GHB due to its properties as a mucous membrane irritant and/or solvent. The most effective way to reduce this is to dilute it with a large meal or drink.
- Stomach cramps
- Motor control loss
- Neurotoxicity - GBL is metabolized into GHB, so it may also be linked with neurotoxicity. Lower dosages have been linked with higher degrees of neurotoxicity than high doses, although this claim can be debated.[8][9][10][11][12]
- Skin flushing
- Dizziness
- Dehydration
- Muscle cramps - Abrupt discontinuation of frequent use can also result in muscle cramps and spasms.
- Optical sliding
- Increased salivation - Increased salivation is very common.
- Increased perspiration - Cold sweats are more common than with GHB.
- Pupil dilation
- Vasodilation
- Headaches
- Seizure - Anecdotal reports suggests that GBL is less prone to seizures than GHB.
Visual effects 
-
- Visual noise - Especially with extended use or during withdrawal, GBL greatly amplifies existing effects of visual noise for long periods of time, also including after images and tinnitus. This is presumably due to a state of excitation within the brain or metabolic acidosis.[citation needed] This is far more prevalent with GBL than GHB.
- Visual acuity suppression
- After images
- Perspective distortions
- Depth perception distortions
- Scenery slicing
- Geometry - Geometry results along with visual noise, especially if the user has had experience with psychedelics or has HPPD
Cognitive effects 
-
- Sleepiness & Wakefulness - At very low dosages, GBL can make one tired, while common doses are primarly wakefullness-promoting. High doses can lead to feelings of being extremely sleepy.
- Analysis suppression
- Anxiety suppression
- Disinhibition
- Cognitive euphoria - GBL is far more euphoric than alcohol or benzodiazepines, more akin to Gabapentinoids.
- Empathy, affection, and sociability enhancement - Unlike alcohol which merely increases sociability through disinhibition, GBL presents strong entactogenic effects which are prominent and well defined although weaker than that of MDMA.
- Thought acceleration or Thought deceleration - Low to common doses are primarly stimulating and one finds themselves constantly generating thoughts.
- Memory suppression - Extended use of GBL can result in memory problems as well as forgetfulness and not being able to differentiate dreams from waking memories.
- Amnesia - Higher doses, especially when sleeping can lead to total amnesia.
- Creativity enhancement - Creativity enhancement is most apparent at low to common doses.
- Compulsive redosing - This is somewhat prevalent due to its short duration.
- Dream potentiation - GBL can have a very pronounced effect on dream recall and vividness. Conversely, discontinuation can result in bad dreams or nightmares. Additionally, intoxication on GBL can lead to one seeing their own dream being manifested, similar to a wake-induced lucid dream (WILD) and simultaneous states of wakefulness and dreaming.
- Introspection
- Rejuvenation
- Motivation enhancement & Motivation suppression - As with other coinciding effects, common doses can lead to greater motivation whilst high doses generally result in lethargy.
- Confusion
- Increased libido - This is a very pronounced and documented effect with GHB, when compared with most other depressants.
- Increased music appreciation
- Spatial disorientation - Disorientation is very common in dark areas.
- Suggestibility enhancement
Auditory effects 
-
- Tinnitus - This is a distinct but significant ringing and/or buzzing in the ears which can sometimes persist for long amounts of time. It typically co-exists with visual noise during extended use or withdrawal. This is far more prevalent with GBL than GHB.
- Auditory distortion
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
GBL is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB, meaning that it is rapidly converted into GHB in the body.
GBL is not considered to be as safe as GHB used responsibly or medicinally, since it is theorized to display additional toxic effects on the body, unlike GHB. The LD50 is above the active dosage, and there is no danger of acute toxicity. However, it can become dangerous when used as a recreational drug or abused. There have been many negative reports from recreational users who have overdosed, combined GBL with alcohol or other drugs, or accidentally dosed themselves unexpectedly or way above the common dosage range.
To avoid a possible overdose of GBL, it is important to start with a low dose and work your way up slowly by increasing the dosage in small increments. One should best use a glass bottle with an integraded 1ml pipette to exactly measure their dosages.
Accidental ingestions of GBL have also occurred due to inadequate storage methods. If GBL is put into a clear liquid, glass, or bottle, it can be easily mistaken for water. It is recommended to clearly label your GBL in writing and dye the liquid with blue food coloring so it no longer resembles a drinkable beverage. It is also recommended to store your GBL in a container that no one would drink out of.
It is strongly recommended that one use harm reduction practices when using this drug.
Neurotoxicity
GBL itself may also produce neurotoxic effects, in addition to the effects of GHB, to which it is converted to by the body.[5]
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[13][9][10][11] These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well.[12]
One study found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells within the hippocampus and in the prefrontal cortex. With once daily doses of 10 mg/kg of GHB, they were decreased by 61% in the hippocampus region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. This paper demonstrates contradicting effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.[14]
Tolerance and addiction potential
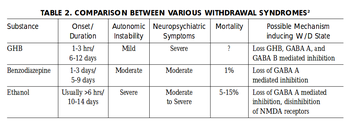
GBL is possibly highly physically and moderately to highly psychologically addictive. The frequent use of GBL can cause withdrawal symptoms similar to those caused by other depressants such as alcohol and benzodiazepines if abruptly discontinued, reportedly with a much faster onset and more severe symptoms when used frequently. Withdrawal effects from GBL build faster than with GHB.[16][17] These symptoms seem to depend on the dosage and the length of time the drug was used for. Light to moderate users often experience anxiety, insomnia, sleep-related problems, and tremors whereas heavy use can cause severe withdrawal symptoms like delirium, psychosis, and hallucinations, which some anecdotal reports suggesting the effects to be more severe than other depressants.[18][15]
GHB, when used medicinally (two medicinal doses per day, four hours apart), creates little to no dependance while the more frequent one uses GHB, it causes exponentially more dependance and withdrawal symptoms. It is though that GBL also shares this trait, although there is no medicinal dose for GBL that is safe.[19]
Although there have been reported fatalities due to GHB/GBL withdrawal, reports are inconclusive and further research is needed.[20]
Tolerance will develop to the sedative-hypnotic effects within several days of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few days or longer of steady dosing, and may necessitate a gradual dose reduction to minimize neurotoxicity from withdrawal. It is proposed that GHB and especially GBL can lead to dependence at a significantly faster rate than longer-acting depressants.
GBL presents cross-tolerance with 1,4-Butanediol and GHB, since GBL and 1,4-Butanediol act as prodrugs for GHB. Cross-tolerance with other depressants such as alcohol, phenibut, baclofen and other GABAB-agonists is likely.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Nitrous - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position. Memory blackouts are likely.
- Amphetamines - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the sedative may overcome the patient and cause respiratory arrest.
- MDMA - Large amounts of GHB/GBL may overwhelm the effects of MDMA on the comedown.
- Cocaine - Stimulants increase respiration rate allowing a higher dose of sedatives. If the stimulant wears off first then the sedative may overcome the patient and cause respiratory arrest.
- Ketamine - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- MXE - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position.
- DXM - Both substances cause ataxia and bring a risk of vomiting and unconsciousness. If the patient falls unconscious while under the influence there is a severe risk of vomit aspiration if they are not placed in the recovery position. This combination is hard to predict.
- PCP - Details of this combination are not well understood but PCP generally interacts in an unpredictable manner.
- Alcohol - Even in very low doses this combination rapidly leads to memory loss, severe ataxia and unconsciousness. There is a high risk of vomit aspiration while unconscious.
- Opioids - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
- Tramadol - The sedative effects of this combination can lead to dangerous respiratory depression.
- Benzodiazepines - The two substances potentiate each other strongly and unpredictably, very rapidly leading to unconsciousness. While unconscious, vomit aspiration is a risk if not placed in the recovery position.
Legal status
- Australia: GBL is a border controlled substance and is illegal to import into Australia without a permit. The importation of a commercial quantity of a border controlled drug (over 1 kg of GBL) is punishable by up to life imprisonment and/or an $825,000 fine.[21]
- Austria: Since January 1, 2012, GBL is illegal to possess, produce and sell under the NPSG (Neue-Psychoaktive-Substanzen-Gesetz Österreich).[citation needed]
- Canada: GBL is a Controlled Substance under Schedule VI of the "Controlled Drugs and Substances Act" in Canada. Schedule VI of the "Controlled Drugs and Substances Act" requires vendors to collect information regarding purchases of GBL. The Act also prohibits the import and export of GBL into or out of Canada classifying it as either an indictable offence punishable with up to 10 years in prison or an offence punishable on summary conviction liable to imprisonment for up to eighteen months.[22] It is not illegal for an individual to possess GBL in Canada.
- Germany: GBL is not listed in the narcotics law, but its distribution is controlled. Possession is not illegal, but may be punished according to the Medicines Act, when intended to be sold for human consumption or synthesis of GHB. In recent years, an increase of GBL consumption has been observed due to the prohibition of GHB.[citation needed]
- Hong Kong: GBL is a dangerous drug controlled under Schedule 1 of the Dangerous Drugs Ordinance, Cap.134 (with exemption clause at Paragraph 16D). Any person who is found to have in his possession of it not in accordance with this Ordinance can be liable, on conviction upon indictment, a fine of HK$1,000,000 and to imprisonment for 7 years.[citation needed]
- Israel: GBL was classified as a proscribed substance from 2007.[23]
- Netherlands: GBL can be freely bought as a cleaning agent. Retailers do not need a licence to sell the substance.[24]
- Poland: GBL is classified as a drug and handling it requires a pharmaceutical license.[25]
- Sweden: GBL is not classified as a drug but as a health-endangering substance. Although recently passed legislation to enter into force on 1 April 2011 will make it possible to handle narcotics for industrial purposes will enable GBL and 1,4-Butanediol to be classified as controlled substances.[26]
- Switzerland: GBL is considered an ester analog of GHB, which would make it illegal according to Buchstabe B. Industrial use however is permitted.[27]
- Turkey: GBL is a classed as drug and is illegal to possess, produce, supply, or import.[28]
- United Kingdom: Because of their legitimate uses, regulation 4B of the 2001 regulations makes it lawful to import, export, produce, supply, offer to supply or possess GBL and 1,4-BD. Except where a person does so knowing or believing that they will be used for the purpose of human ingestion.[29][30]
- United States: GBL is regulated as a List 1 controlled chemical. As a GHB analog, it is treated as a controlled substance under Schedule I of the "Controlled Substances Act" if intended for human consumption.[31]
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ Erowid 1,4-butanediol Vault : Storage : 14b and GBL May Dissolve Some Plastics, 2001
- ↑ Vose, J., Tighe, T., Schwartz, M., Buel, E. (September 2001). "Detection of gamma-butyrolactone (GBL) as a natural component in wine". Journal of Forensic Sciences. 46 (5): 1164–1167. ISSN 0022-1198.
- ↑ https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/100526/21-2009.pdf
- ↑ 5.0 5.1 5.2 Teiber, J. F., Draganov, D. I., Du, B. N. L. (September 2003). "Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3". Biochemical Pharmacology. 66 (6): 887–896. doi:10.1016/S0006-2952(03)00401-5. ISSN 0006-2952.
- ↑ https://drugs-forum.com/threads/important-ghb-gbl-addiction-withdrawal.43390/
- ↑ Rhodium GHB Synthesis FAQ
- ↑ Sircar, R., Basak, A. (1 December 2004). "Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning". Pharmacology Biochemistry and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ 9.0 9.1 García, F. B., Pedraza, C., Arias, J. L., Navarro, J. F. (August 2006). "[Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats]". Psicothema. 18 (3): 519–524. ISSN 0214-9915.
- ↑ 10.0 10.1 Sircar, R., Basak, A., Sircar, D. (October 2008). "γ-Hydroxybutyric Acid-Induced Cognitive Deficits in the Female Adolescent Rat". Annals of the New York Academy of Sciences. 1139 (1): 386–389. doi:10.1196/annals.1432.044. ISSN 0077-8923.
- ↑ 11.0 11.1 Pedraza, C., García, F. B., Navarro, J. F. (October 2009). "Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats". The International Journal of Neuropsychopharmacology. 12 (09): 1165. doi:10.1017/S1461145709000157. ISSN 1461-1457.
- ↑ 12.0 12.1 Sircar, R., Basak, A. (December 2004). "Adolescent gamma-hydroxybutyric acid exposure decreases cortical N-methyl-D-aspartate receptor and impairs spatial learning". Pharmacology, Biochemistry, and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ Sircar, R., Basak, A. (1 December 2004). "Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning". Pharmacology Biochemistry and Behavior. 79 (4): 701–708. doi:10.1016/j.pbb.2004.09.022. ISSN 0091-3057.
- ↑ Pedraza, C., García, F. B., Navarro, J. F. (October 2009). "Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats". The International Journal of Neuropsychopharmacology. 12 (09): 1165. doi:10.1017/S1461145709000157. ISSN 1461-1457.
- ↑ 15.0 15.1 GHB Withdrawal Syndrome | Texas Commission on Alcohol and Drug Abuse | https://www.erowid.org/chemicals/ghb/ghb_addiction2.pdf
- ↑ Kim, S. Y., Barker, J. C., Anderson, I. B., Dyer, J. E., Earnest, G., Blanc, P. D. (2008). "Systematic Assessment of Gamma Hydroxybutyrate (GHB) Effects During and After Acute Intoxication". The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 17 (4): 312–318. doi:10.1080/10550490802138988. ISSN 1055-0496.
- ↑ Carter, L. P., Pardi, D., Gorsline, J., Griffiths, R. R. (1 September 2009). "Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): differences in characteristics and misuse". Drug and alcohol dependence. 104 (1–2): 1–10. doi:10.1016/j.drugalcdep.2009.04.012. ISSN 0376-8716.
- ↑ Dyer, J. E., Roth, B., Hyma, B. A. (February 2001). "Gamma-hydroxybutyrate withdrawal syndrome". Annals of Emergency Medicine. 37 (2): 147–153. doi:10.1067/mem.2001.112985. ISSN 0196-0644.
- ↑ https://drugs-forum.com/threads/important-ghb-gbl-addiction-withdrawal.43390/page-4
- ↑ Galloway, G. P., Frederick, S. L., Staggers, F. E., Gonzales, M., Stalcup, S. A., Smith, D. E. (January 1997). "Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence". Addiction. 92 (1): 89–96. doi:10.1111/j.1360-0443.1997.tb03640.x. ISSN 0965-2140.
- ↑ Law and Justice Legislation Amendment (Serious Drug Offences and Other Measures) Act 2005 No. 129, 2005
- ↑ Branch, L. S. (2022), Consolidated federal laws of Canada, Controlled Drugs and Substances Act
- ↑ section 7c of chapter B of part A of the 1st appendix of the Dangerous Drugs Act 1973 http://www.nevo.co.il/Law_word/law01/P170_001.doc
- ↑ "Verkopers schoonmaakmiddel verdienen fors aan partydrug GHB". Trouw (in Dutch). 11 April 2012. Retrieved 11 April 2012.
- ↑ "undefinedKomunikat Nr 3/2016 Głównego Inspektora Farmaceutycznego w sprawie GBL (y-Butyrolaktonu)" (in Polish). 11 April 2021. Retrieved 11 April 2021.
- ↑ Socialutskottets betänkande 2010/11:SoU5 - Riksdagen
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ "Karar Sayısı: 2016/9019" (PDF). Resmî Gazete, Sayı: 29790 (in Turkish). June 22, 2016.
- ↑ http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/100526/21-2009.pdf
- ↑ "UK Statutory Instrument 2011 No. 448". 2011-02-18.
- ↑ Information Bulletin: GHB Analogs; GBL, BD, GHV, and GVL