Talk:4C-D
| Summary sheet: 4C-D |
| 4C-D | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | 4C-D, ARIADNE, dimoxamine, α-ethyl-2C-D, α-ethyl-DOM, BL-3912 | ||||||||||||||||||||||||||||||||
| Substitutive name | 4-Methyl-2,5-dimethoxy-alpha-ethylphenethylamine | ||||||||||||||||||||||||||||||||
| Systematic name | 1-(2,5-Dimethoxy-4-methylphenyl)butan-2-amine | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||
| Chemical class | Phenisobutylamine | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
4-Methyl-2,5-dimethoxy-alpha-ethylphenethylamine (also known as ARIADNE, Dimoxamine and more commonly as 4C-D) is a synthetic psychedelic that produces a mixture of psychedelic, entactogenic and stimulant effects when administered.
4C-D was first synthesized by Alexander Shulgin and documented in his 1991 book PiHKAL ("Phenethylamines I Have Known and Loved").[1] Shulgin has researched dosages up to 32 milligrams, although anecdotal reports suggest that the dosage range for 4C-D is generally higher than what Shulgin documented. Shulgin describes 4C-D as not a true psychedelic and that it possesses anti-depressant effects. It has been researched as a potential therapeutic agent for depression and loss of motivation due to its mood-lifting effects.[citation needed]
4C-D has been scarcely distributed as a gray area research chemical by online vendors. Many anecdotal reports suggest that the psychedelic effects begin to unfold at the higher dose range. The dosage levels of 4C-D are therefore not fully clear, and one should start at a lower dose when researching this compound. It is highly advised to use harm reduction practices if using this substance.
History and culture
4C-D was once patented as a therapeutic aid in elderly people for restoring motivation under the name Dimoxamine.[citation needed]
Chemistry
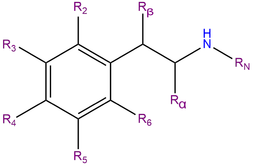
4C-D, or 4-Methyl-2,5-dimethoxy-alpha-ethylphenethylamine, is a substituted phenylisobutylamine featuring a phenyl ring bound to a sec-butylamino chain. 4C-D contains methoxy functional groups CH3O- attached to carbons R2 and R5 as well as a methyl group attached to carbon R4 of the phenyl ring.
4C-D belongs is an alpha-ethyl substituted phenethylamine and can be considered the alpha-ethyl analog of 2C-D and DOM.
Pharmacology
4C-D's psychedelic effects are believed to come from its efficacy at the 5-HT2A receptor as a partial agonist. However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
In a forum thread,[2] users have proposed that 4C-D may act as a MAOI, based on structural similarities to 5-MeO-7-Me-AET or 7-Me-AET, which has been shown to act as a strong MAOI and serotonin releasing agent. There is no evidence to support this claim that 4C-D may share these properties as well, but it should be taken into consideration when choosing to combine this substance with other monoaminergics.
Subjective effects
The head space of 4C-D is relatively mild at lower dosages, and it has been noted for its unusual mood-lifting and motivational effects.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - In terms of its effects on the physical energy levels of the user, the 4C-D experience begins with mild sedation that gives way to moderate stimulation as the experience progresses. It lacks the forceful energetic push associated with most psychedelic phenethylamines.
- Spontaneous physical sensations - The "body high" of 4C-D can be described as a pleasurable yet complex, all-encompassing comfort sensation. This maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Bodily control enhancement - Whilst at lower doses one may find that their bodily control is enhanced, at higher dosages it seems to become suppressed.
- Tactile enhancement
- Temperature regulation suppression
- Increased heart rate
- Increased blood pressure
- Dehydration
- Nausea
- Pupil dilation
Visual effects 
-
The visual effects of 4C-D tend to be weak at lower dosages and begin to unfold at higher ones. It is purported to resemble many other psychedelics with it having features such as the patterning of psilocin and DOM, the mild waviness of 2C-D as well as visual distortions reminiscent of 3C-E.
Enhancements
Distortions
- Drifting (melting, flowing, breathing and morphing) - In comparison to other psychedelics, this effect can be described as simplistic in complexity, slow and smooth in motion, static in appearance and realistic in style.
- Tracers
- After images
- Colour shifting
- Colour tinting
- Symmetrical texture repetition
- Recursion
- Diffraction
Geometry
The visual geometry of 4C-D be described as more similar in appearance to that of psilocin or 2C-D than that of 2C-B, LSD or mescaline. It can be comprehensively described as structured in its organization, organic in style, intricate in complexity, large in style, slow and smooth in motion, colorful in scheme, bright in color, blurred in its edges and equally rounded and angular in its corners. While the final level of 4C-D geometry has yet to be formally confirmed, it seems more likely that it would result in states of level 8B visual geometry over level 8A.
Hallucinatory states
- Transformations
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - In comparison to other psychedelics such as LSD, 4C-D is low in hallucinations embedded within visual geometry. However, when it does occur this particular effect commonly contains hallucinations with scenarios, settings, concepts and autonomous entity contact. They are more common within dark environments and can be described as internal in their manifestation, lucid in believability, and interactive in style.
- External hallucination (settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - 4C-D is capable of external hallucinations embedded within visual geometry. This particular effect commonly contains hallucinations with scenarios, settings, concepts and autonomous entity contact. They can be described as external in their manifestation, lucid in believability, and fixed in style.
Cognitive effects 
- The headspace of 4C-D is reported to be very mild and mostly clearheaded at low to moderate dosages in a fashion similar to 2C-D.
Auditory effects 
Multi-sensory effects 
-
- Synaesthesia - In its fullest manifestation, this is a very rare and non-reproducible effect. Increasing the dosage can increase the likelihood of this occurring, but seems to only be a prominent part of the experience among those who are already predisposed to synaesthetic states.
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
The toxicity and long-term health effects of recreational 4C-D use do not seem to have been studied in any scientific context and the exact toxic dose is unknown. This is because 4C-D is a research chemical with very little history of human usage.
Anecdotal evidence from those within the community who have tried 4C-D suggests that there are no negative health effects attributed to simply trying the substance by itself at low to moderate doses and using it very sparingly (but nothing can be completely guaranteed). Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
It is strongly recommended that one use harm reduction practices when using this substance.
Tolerance and addiction potential
4C-D is not habit-forming and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of 4C-D is built almost immediately after ingestion. After that, it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). 4C-D presents cross-tolerance with all psychedelics, meaning that after the consumption of 4C-D all psychedelics will have a reduced effect.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Lithium - Lithium is commonly prescribed for the treatment of bipolar disorder. There is a large body of anecdotal evidence that suggests taking it with psychedelics significantly increases the risk of psychosis and seizures. As a result, this combination is strictly discouraged.
- Cannabis - Cannabis may have an unexpectedly strong and unpredictable synergy with the effects of 4C-D. Caution is advised with this combination as it can significantly increase the risk of adverse psychological reactions like anxiety, paranoia, panic attacks, and psychosis. Users are advised to start off with only a fraction of their normal cannabis dose and take long breaks between hits to avoid unintentional overdose.
- Stimulants - Stimulants like amphetamine, cocaine or methylphenidate affect many parts of the brain and alter dopaminergic function. This combination can increase the risk of anxiety, paranoia, panic attacks, and thought loops. This interaction may also result in an elevated risk of mania and psychosis.[citation needed]
- Tramadol - Tramadol is well-documented to lower the seizure threshold[3] and psychedelics may act to trigger seizures in susceptible individuals.[citation needed]
Legal status
- Germany: 4C-D is controlled under the NpSG (New Psychoactive Substances Act) as of November 26, 2016.[4][5] Production and import with the aim to place it on the market, administration to another person and trading is punishable. Possession is illegal but not penalized.[6]
- Switzerland: 4C-D can be considered a controlled substance as a defined derivative of Phenethylamine under Verzeichnis E point 130. It is legal when used for scientific or industrial use.[7]
- United Kingdom: 4C-D is a Class A drug in the United Kingdom as a result of the phenethylamine catch-all clause.[8]
- United States: 4C-D is technically not scheduled in the United States but could be considered an analogue of 2C-D or DOM and may therefore be considered a Schedule I drug under the Federal Analogue Act.[citation needed]
See also
External links
Discussion
References
- ↑ https://erowid.org/library/books_online/pihkal/pihkal008.shtml
- ↑ https://www.bluelight.org/xf/threads/4c-d-ariadne.859113/
- ↑ Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). "Dose-independent occurrence of seizure with tramadol". Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. ISSN 1556-9039.
- ↑ "Gesetz zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe" (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 10, 2019.
- ↑ "Anlage NpSG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 10, 2019.
- ↑ "§ 4 NpSG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 10, 2019.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ United Kingdom. (1977). Misuse of Drugs Act 1971 (S.I. 1977/1243). London: The Stationery Office Limited. Retrieved July 5, 2017, from http://www.legislation.gov.uk/uksi/1977/1243/made