Methallylescaline
| Summary sheet: Methallylescaline |
| Methallylescaline | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | Methallylescaline, MAL | ||||||||||||||||||||||||||||||||
| Substitutive name | 4-methallyloxy-3,5-dimethoxyphenethylamine | ||||||||||||||||||||||||||||||||
| Systematic name | 2-(3,5-dimethoxy-4-[(2-methylprop-2-en-1-yl)oxy]phenyl)ethanamine | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||
| Chemical class | Phenethylamine | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
4-Methallyloxy-3,5-dimethoxyphenethylamine (also known as methallylescaline and MAL) is a synthetic psychedelic substance of the phenethylamine chemical class. It is reported to produce hallucinogenic and stimulant effects comparable to that of other mescaline analogs (e.g. allylescaline, proscaline, and escaline) when administered.
The effects of methallylescaline were first described by Alexander Shulgin in his book PiHKAL: A Chemical Love Story. He lists the dosage range as 40mg to 60mg orally and describes the duration of action to be 8 - 12 hours.[1] Anecdotal reports portray that methallylescaline bears the most similarity to mescaline, although higher dosages can produce a strong body load.
Very little data exists about the pharmacological properties, metabolism, and toxicity of methallylescaline, and it has little history of human usage. It is highly advised to use harm reduction practices if using this substance.
Chemistry
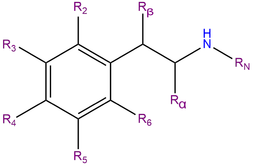
Methallylescaline, or 4-methallyloxy-3,5-dimethoxyphenethylamine, is a substituted phenethylamine featuring a phenyl ring bound to an amino (NH2) group through an ethyl chain. Methallylescaline contains two methoxy functional groups (CH3O-) which are attached to carbons R3 and R5 of the phenyl ring.
Methallylescaline is substituted at R4 with a methallyloxy chain. This three carbon chain consists of a R2 methyl substituted allyl group with a double bond on the terminal carbon. This chain is connected to the phenyl ring at R4 through an ether (oxygen) bridge.
Pharmacology
Methallylescaline likely acts as a 5-HT2A partial agonist. The psychedelic effects are believed to come from methallylescaline's efficacy at the 5-HT2A receptors. However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
Subjective effects
 |
This subjective effects section is a stub. As such, it is still in progress and may contain incomplete or wrong information. You can help by expanding or correcting it. |
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Stimulation - In terms of its effects on the physical energy levels of the user, methallylescaline is usually considered to be very energetic and stimulating. For example, when taken in any environment it will usually encourage physical activities such as running, walking, climbing or dancing. In comparison, other more commonly used psychedelics such as psilocin are generally sedating and relaxed.
- Spontaneous bodily sensations - The "body high" of methallylescaline can be described as proportionally intense in comparison to its accompanying visual and cognitive effects. It is manifested in a number of forms including an intense soft, warm glow that grows over the body and is capable of becoming extremely physically euphoric. This is most similar to MDMA and MDA and is consistently manifested throughout the experience. This is contrasted by an intensely pleasurable yet sharp, cold electric tingling sensation which moves up and down the body. This is most similar to mescaline and is also consistently manifested throughout the trip. The final physical effect noticed throughout the experience is an intense energetic pins and needles sensation that manifests itself in the form of a continuously shifting and tingling sensation that travels up and down the body in spontaneous waves. This is most similar to 2C-I and is not entirely consistent throughout the trip.
- Perception of bodily lightness
- Physical euphoria - Relative to other psychedelics, methallylescaline has been noted for the bodily or physically euphoric aspect that is vaguely reminiscent of other phenethylamines like MDA.
- Nausea - Nausea is commonly reported when consumed in moderate to high doses and either passes instantly once the user has vomited or gradually fades by itself as the peak sets in.
- Muscle cramp - This is uncommon, but is more likely to occur if one is malnourished, dehydrated, or engaged in strenuous activity.
- Tactile enhancement - Feelings of enhanced tactile sensations are consistently present at moderate levels throughout most methallylescaline experiences. If Level 8A geometry is reached, an intense sensation of suddenly becoming aware of and being able to feel every single nerve ending across a person's entire body all at once is consistently present.
- Appetite suppression
- Difficulty urinating - A notable side effect of methallylescaline is the occurrence of urinary retention, where users may experience difficulty urinating despite a frequent or urgent urge to do so. This effect can result in discomfort and frustration, as the physical sensation of needing to urinate persists without successful relief. It tends to be more pronounced at higher doses and may continue for several hours after the peak effects. While not universally experienced, it can be a significant concern for some users, especially if they are prone to bladder discomfort or if the urge to urinate becomes overwhelming without being able to find relief. Urinary retention can be managed with alpha blockers such as tamsulosin, which relax the muscles and promote urination.
- Increased heart rate
- Increased libido
- Muscle contractions
- Pupil dilation
- Excessive yawning
Visual effects 
-
Enhancements
Distortions
- Drifting (melting, breathing, morphing and flowing)
- Colour shifting
- Depth perception distortions
- Perspective distortions
- Symmetrical texture repetition
- Tracers
- After images
- Brightness alteration
- Diffraction
Geometry
The visual geometry encountered on methallylescaline can be described as similar in appearance to that of ayahuasca or psilocin in the sense that its geometry is structured in its organization as well as natural and organic in style. However, in terms of its bright color palette and sharp edges, it is also similar to that of LSD, 2C-B and 2C-I.
The geometry can be comprehensively described as:
- Organic in geometric style
- Equally abstract and algorithmic in appearance
- Intricate in complexity
- Structured in its organization
- Fast in speed
- Smooth in motion
- Equally large and small in appearance - It has a variable size that spontaneously changes between large and small in appearance.
- Multicoloured in scheme
- Glossy in colour
- Sharp and angular in its corners
- Level 8B - While the geometry produced by mescaline has yet to be fully characterized, the geometry gives off certain attributes which are significantly more likely to result in states of level 8A visual geometry over level 8B at higher doses.
Hallucinatory states
Cognitive effects 
Auditory effects 
Experience reports
There are currently 0 experience reports describing the effects of this substance in our experience index. You can also submit your own experience report using the same link.
Additional experience reports can be found here:
Toxicity and harm potential
The toxicity and long-term health effects of recreational methallylescaline use do not seem to have been studied in any scientific context and the exact toxic dose is unknown. This is because methallylescaline is a research chemical with very little history of human usage.
Anecdotal evidence suggests that there are no negative health effects attributed to simply trying the substance by itself at low to moderate doses and using it very sparingly (but nothing can be completely guaranteed). Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
It is strongly recommended that one use harm reduction practices when using this substance.
Tolerance and addiction potential
Methallylescaline is not habit-forming, and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of methallylescaline is built almost immediately after ingestion. After that, it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). Methallylescaline presents cross-tolerance with all psychedelics, meaning that after the consumption of methallylescaline all psychedelics will have a reduced effect.
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
- Canada: Methallylescaline is not listed under the Controlled Drugs and Substances Act, so it is technically not illegal.[citation needed]
- Germany: Methallylescaline is controlled under the NpSG[2] (New Psychoactive Substances Act) as of November 26, 2016.[3] Production and import with the aim to place it on the market, administration to another person, placing it on the market and trading is punishable. Possession is illegal but not punishable.[4][5] The legislator considers it possible that orders of methallylescaline are punishable as an incitement to place it on the market.[6]
- Switzerland: Methallylescaline is a controlled substance specifically named under Verzeichnis E.[7]
- United Kingdom: Methallylescaline is illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26th, 2016.[8]
- United States: Methallylescaline is unscheduled in the United States, but may be considered an analogue of mescaline under the Federal Analogue Act.[citation needed]
See also
External links
Discussion
References
- ↑ Alexander Shulgin; Ann Shulgin (1991). "#99. MAL". PiHKAL: A Chemical Love Story. United States: Transform Press. ISBN 0963009605. OCLC 1166889264.
- ↑ "Anlage NpSG" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Gesetz zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe" (PDF). Bundesgesetzblatt Jahrgang 2016 Teil I Nr. 55 (in German). Bundesanzeiger Verlag (published November 25, 2016). November 21, 2016. pp. 2615–2622. ISSN 0341-1095. OCLC 1004462279.
- ↑ "§ 4 NpSG" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "§ 3 NpSG" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Gesetzentwurf der Bundesregierung: Entwurf eines Gesetzes zur Bekämpfung der Verbreitung neuer psychoaktiver Stoffe" (PDF) (in German). Deutscher Bundestag. May 30, 2016. p. 20. Drucksache 18/8579.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ "Psychoactive Substances Act 2016". UK Government. Retrieved January 1, 2020.