Talk:Ecgetrin
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |
| Summary sheet: Ecgetrin |
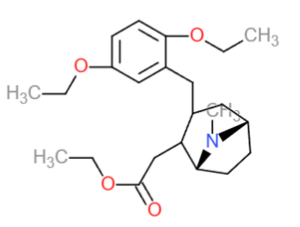
Ecgetrin (also known as Ecgedetrine) is a research chemical and synthetic tropane alkaloid stimulant that is from a similar class to that of Scopolamine, Hyoscyamine or Cocaine but lacks one of the ester/ether/oxygen moieties. It is of the substituted tropanes class. Ecgetrin is predicted to be a dopamine reuptake inhibitor and is structurally related to Troparil and while it has stimulating effects on the CNS, Ecgetrin, as a Troparil or desoxyphenyl type tropane compound (2,5-diethoxy phenyl-methyl tropane-2-acetyllethane) is reported to not effect the sympathetic nervous system in the same way benzoyl tropanes historically have been. It in general is a more balanced reuptake inhibitor, potentially with affinity as an agonist ligand of serotonin and dopamine because of the ethoxy ether moities in its internal structure, as well as the less acidic nature of the ethylcarboxyl moiety at the tropane 2-position, more common in agonists versus reuptake inhibitors. Also, because the serotonin-dopamine activity is more at balance with Ecgetrin than other tropane stimulants and it does not have a high reactivity with most ions it is generally, of non-esterified aromatic tropanes thus less likely to cause adverse or specific physical body loads or abnormal physical sensations or experiences.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Chemistry
This chemistry section is incomplete. You can help by adding to it. |
Pharmacology
 |
This pharmacology section is incomplete. You can help by adding to it. |
Subjective effects
Ecgetrin is reported to have a much more rapid onset and lower half-life when vaporized or smoked. Intranasal
 |
This subjective effects section is a stub. As such, it is still in progress and may contain incomplete or wrong information. You can help by expanding or correcting it. |
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠. {{effects/base
|
Physical effects 
- If applicable, a brief paragraph summary of the substance's physical effects may be included here. You may select physical effects to add below here.
|
Cognitive effects 
Experience reports
No experience reports are available as of yet
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
It is strongly recommended that one use harm reduction practices when using this substance. Since it has cocaine-like effects, it would be wise to assume that it has similar toxicity. Start at low dosages (5-10mg) and work your way up from there.
Lethal dosage
Tolerance and addiction potential
Dangerous interactions
 |
This dangerous interactions section is a stub. As such, it may contain incomplete or invalid information. You can help by expanding upon or correcting it. |
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
See also
External links
(List along order below)
- SUBSTANCE (Wikipedia)
- SUBSTANCE (Erowid Vault)
- SUBSTANCE ([PiHKAL or TiHKAL] / Isomer Design)
Literature
- APA formatted reference
Please see the citation formatting guide if you need assistance properly formatting citations.
References