Talk:Dream potentiation
Neurological Analysis
REM is cholinergic and wakefulness is aminergic
"Pompeiano and Valentinuzzi demonstrated that the [PGO: Ponto-geniculo-occipital] waves could be potentiated by systemic physostigmine, indicating that acetylcholine might play a role in their generation. That the aminergic neuromodulator, serotonin, might hold PGO waves in inhibitory check was suggested by Raymond Cespuglio, who cooled the midline raphe system with a thermode and thereby released torrents of PGO waves. A similar conclusion was reached by Dana Brooks, whose parasagittal pontine brain stem knife cuts released ipsilateral waves and eye movements. It thus looked as if the PGO system, like REM itself, was cholinergic (on) and aminergic (off).
Confirmation of the cholinergic enhancement and aminergic inhibition of PGO waves and REM sleep rests on studies using drugs that promote or impede neurotransmission in these systems; see (Hobson, 2009). These studies illustrate the sophisticated chemical control of REM by the brain. One surprising observation is the absence of a functional deficit, even after prolonged suppression of REM caused by drugs that inhibit MAO (monoamine oxidase) and thus enhance aminergic inhibition by preventing the enzymatic breakdown of NE and 5-HT. A possible explanation of this paradox is that these drugs do the work of REM by artificially boosting the aminergic system, normally rested in REM.
Another finding of great interest is the uncoupling of PGO Waves and REM caused by the microinjection of cholinergic agonists into the burst cell zone of the far-lateral pons (Silberman et al., 1980; Datta et al., 1992). It is not surprising that this experimental intervention immediately triggers ipsiversive eye movements and large ipsilateral PGO waves, but it is remarkable that no increase in REM is observed until 24 h later. This effect lasts for ten days and cannot be due to the persistent presence of the drug, leading to the hypothesis that cholinergic regulation of REM is in the far-lateral pons, while the trigger zone is more paramedian in a region itself devoid of cholinergic neurons."
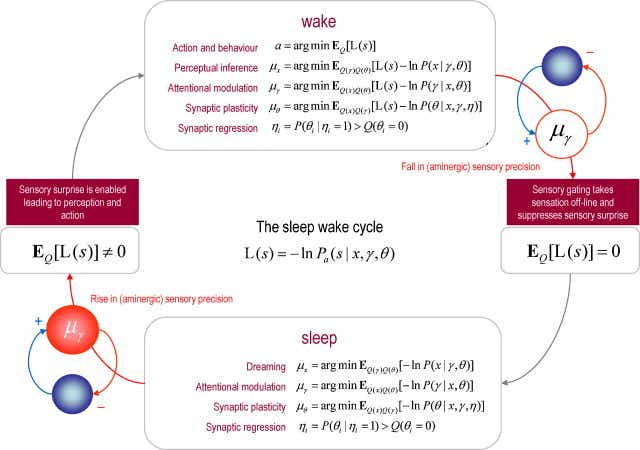
"Fig. 5. This figure shows how the reciprocal interactions between cholinergic and aminergic systems entrain perceptual processes during the sleep wake cycle. Aminergic modulation plays a critical role in gating or enabling precise sensory information to drive action and perception. When this modulatory gating suppresses sensory information, the brain's optimization processes are no longer informed by precise sensory prediction errors and change quantitatively. The implicit optimization processes during waking (upper panel) and sleep (lower panel) are based on free energy minimization, using the generative models described in the previous two figures. The free energy principle requires all internal brain states encoding posterior beliefs or conditional expectations to minimize free energy. The particular updates shown in this figure are based upon a standard variational Bayesian procedure (Beal, 2003) that allow us unpack the various processes involved. For every quantity in the generative model (see Fig. 3) there is a corresponding update. These updates are computed using conditional expectations denoted by E_Q[•]. Here, L(s) = -lnP_a(s|x,γ,θ) is sensory surprise and is just sensory prediction error times its precision. Sensory surprise is effectively turned off during sleep because the precision of sensory prediction errors is reduced. Neurobiologically, we assume this is mediated by circadian fluctuations in aminergic neurotransmission (pink circles). The text describes, briefly, the biophysical and neurobiological processes that can be associated with each of the updates. These are largely the same in sleep and wake, with the exception of action that depends exclusively on sensory surprise (that is absent during sleep). We have included priors governing the presence or absence of a particular connection in these updates. Minimizing the free energy of these priors is formally identical to model selection using the Savage-Dickey density ratio: see (Friston and Penny, 2011) for details. Effectively, this removes or prunes redundant model parameters (synaptic connections) to reduce model complexity and minimize free energy. The optimization of the parameters can be seen in the same light, because these effectively minimize the complexity of empirical priors on hidden states." Figures are under a creative commons license.
"Clearly, the emergence of PGO waves during REM sleep presupposes that the oculomotor system acquires a selective neuromodulatory boost during periods of REM sleep that we presume is mediated by cholinergic afferents. This fits nicely with the six-fold increase in the excitability of midbrain PGO burst cells in REM sleep, relative to waking (Nelson et al., 1983). In summary, perceptual inference can be regarded as a response to exteroceptive (visual) prediction errors during wakefulness, while the same responses are elicited by proprioceptive (oculomotor) prediction errors during sleep. Put simply, waking percepts are driven by the need to explain unpredicted visual input, while dreaming percepts are driven by the need to explain unpredicted oculomotor input. This interpretation follows directly from the notion of the brain as a generative model of its sensorium that necessarily entails bimodal predictions of exteroceptive and proprioceptive sensations to synthesize its virtual reality (Hobson, 2009)."[1]
Further Reading
REM sleep and dreaming: towards a theory of protoconsciousness
References
- ↑ Hobson, J. A., & Friston, K. J. (2012). Waking and dreaming consciousness: neurobiological and functional considerations. Progress in neurobiology, 98(1), 82-98. https://doi.org/10.1016/j.pneurobio.2012.05.003