Isopropyl alcohol extraction (purification, recrystallization, or blotting)
This guide is provided for informational and educational purposes only. We do not encourage you to break the law and cannot claim any responsibility for your actions. |
An isopropyl alcohol extraction (also known as IPA extraction) is a basic chemistry procedure that is commonly used to extract and recrystallize many different substances, typically to increase purity.
Examples include removing pill binders from substances such as methylphenidate or adderall, or to remove cuts from street drugs (e.g. baking soda from cocaine). It can also be used to recrystallize drugs like ketamine or even blotting some substances.
This article covers the basics of how to safely and effectively perform an IPA extraction.
Risks and hazards
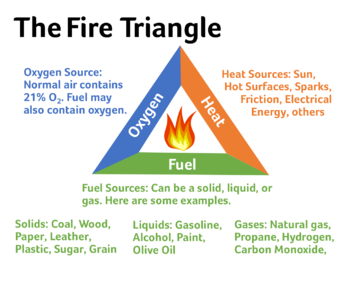
Be incredibly careful when working with isopropyl alcohol or any other flammable substances. High temperatures, sparks, or open flames can cause fires or explosions since isopropyl alcohol emits flammable vapours!
Additionally, breathing isopropyl alcohol vapours is dangerous and these experiments should be done in a well-ventilated area with proper protective equipment.
In order for an explosive event to occur there needs to be an ignition source or spark. This spark can come from common sense items such as a lighter or pilot light on a stove, but sparks can also occur in less obvious ways such as static electricity , certain materials grinding against each other, and electric components found on stoves or car components.[1]
Electric sparks can be created simply by wearing clothes - the common "shock" one experiences after shuffling around with socks on and touching a metal object, along with the sparks created by the compressors in most common kitchen freezers, is sufficient to cause an explosive event.
The flammability of a gas depends on its concentration in the atmosphere. If the concentration of isopropyl alcohol falls in this range and there is a spark present, there will be an ignition and an explosive event[2].
Materials
Required
- Isopropyl alcohol (the higher % is better) (you can salt isopropyl alcohol for a higher purity)
- A clear glass container that can hold the substance + isopropyl alcohol and be stirred without spills
- A large flat glass drying surface, or a glass baking dish for larger quantities
If removing pill binders/cuts
- Filter paper (lab grade 1 will suffice) or coffee filter
- A second glass container to hold filtered solution
- Funnel to hold filter paper over the second glass container
If blotting
- Fluid measuring device (syringe, graduated cylinder, or measuring cup depending on how much solution is created)
- Blotting paper (or watercolor paper works as a cheap alternative)
- Make sure you keep track of exactly how much solvent is used if blotting since you need to know to determine the blotted dosages at the end.
Optional
- Blowdryer / Fan
Steps
Step 1: Determine if the substance is soluble in isopropyl alcohol
To find out if a substance is soluble in alcohols use PubChem or similar chemical properties documentation. Navigate to chemical and physical properties and then scroll down to the solubility section. Some substances do not have a solubility for alcohol listed but still are soluble. You can always just google it and use a large amount of solvent to ensure complete dissolution.
Step 2: Crushing pills (optional):
If pills are being used, they should be thoroughly crushed into a fine powder before proceeding.
Step 3: Combining the substance and the isopropyl alcohol
For this example, we will dissolve 100mg of etizolam. Etizolam is soluble in isopropyl alcohol so for easy measurement we will start with 100ml of isopropyl alcohol, giving it a 1mg/ml ratio after dissolving. First 100mg of etizolam is put into the stirring container then 100ml of isopropyl alcohol is slowly poured over the etizolam powder.
Step 4: Dissolving the substance
The solution is stirred until all of the etizolam is dissolved. Some substances take awhile to fully dissolve and light heat (such as a hair dryer) can be applied to the outside of the container to help the substance dissolve (high temperatures can crack the glass, light the isopropyl alcohol on fire, or burn the substance). In the case where cuts or pill binders are present, there will be undissolved residue left over in the bottom of the container. It is recommended for cuts/pill binder that this step is repeated multiple times to maximize the amount of target substance extracted.
Step 5: Filtering the solution (optional)
The solution is poured through the filter paper into the other glass container and then once the solution has filtered through the paper, a small amount of isopropyl alcohol is poured through the filter paper to wash any loose substances through (also keep track of how much was used to wash if blotting). Once the filter paper has stopped dripping, remove it from the funnel and gently brush any of the remaining particles back into the first glass and repeat step 3 (you can use less solvent this time, but keep track of it if blotting).
Step 6: Retrieving powder, crystal, or blotter
Once you are satisfied with the number of filtrations done you have two options:
- Step 6-A. Evaporate all solution to retrieve the purified substance
- Step 6-B. Blot the substance
Either method requires all the solvent to be dissolved before the process is complete. Don't attempt to store the substance as a solution unless it is stored in an air tight container as isopropyl alcohol will evaporate in air. Also, some substances aren't stable when stored in isopropyl alcohol for a long time and may break down over time.
Step 6-A: Recovering the substance
This will result in the substance being recovered from the solvent as either a powder or crystal. The solution is gently poured onto the flat surface and allowed to air dry. If the substance normally crystallizes and is poured into a baking dish, crystals will form on the bottom of the dish as the isopropyl alcohol evaporates (a light heat source such as a hair dryer can be used to speed up the process, but faster evaporation will prevent crystal formation).
Crystal formation can also be increased by 'seeding' it. To do this, add some already crystallized substance to the solution. The solution must be at a point where it no longer will dissolve any more of the substance. You can wait until enough has evaporated that you start seeing some of the substance forming at the bottom of the container.
Once all the isopropyl alcohol is evaporated (there is no more visible liquid, substance is dry and there is no longer an alcohol smell) you are done and all that's left is your substance (and possibly some cuts that are soluble in isopropyl alcohol but some common cuts like baking soda will be removed, resulting in higher purity). The substance can be removed by breaking it up into chunks or scratching it off with a razor blade (be careful not to break the glass container its stored in).
Step 6-B: Blotting the substance
This requires the substance to be high potency and very soluble. It also requires that the exact amount of substance and solvent are known The potency of each blotter will be equal to the amount of substance per unit of solution multiplied by the amount of solution on each blotter. For example, 100mg of etizolam powder was dissolved in a total of 100ml isopropyl alcohol; this means that there is 100ml of solution at 1mg/ml. For this example, 300g/m^2 watercolor paper was used and 1/2 inch squares would hold approximately 0.5ml of solution.
Each 1/2 inch square is drawn onto the paper using a small amount of food coloring (anything can be used to define the squares, but just make sure its non-toxic). Using an accurate measuring device (such as a 1ml syringe), the solution is drawn into the barrel 0.5ml at a time then slowly and evenly spread out over each square of paper.
As each square has solution added, the solution will remain in a bubble on top of the square then slowly spread out, using the tip of the syringe move the solution around so it conforms to the shape of the square and does not extend into the other squares. This is a long process but makes dosing high potency substances much easier.
Due to the long time required, it may be necessary to keep the solution in an air tight container while blotting to prevent evaporation if a significant amount of IPA evaporates before blotting. Each blotter is stronger than the last (minuscule difference but might be noticeable between the first and last blotters.)
References
- ↑ "Fire Protection and Prevention", OSHA https://www.osha.gov/dte/grant_materials/fy09/sh-18796-09/fireprotection.pdf
- ↑ Flammability of Gasses http://people.clarkson.edu/~wwilcox/Design/flamlim2.pdf