Talk:Triazolam
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |

Fatal overdose may occur when benzodiazepines are combined with other depressants such as opiates, barbiturates, gabapentinoids, thienodiazepines, alcohol or other GABAergic substances.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: Triazolam |
| Triazolam | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||
| Common names | Halicon, Triazolam | ||||||||||||||||||||||||||||||
| Substitutive name | Triazolam | ||||||||||||||||||||||||||||||
| Systematic name | 8-Chloro-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine | ||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||
| Chemical class | Benzodiazepine | ||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||
Triazolam (trade name Halcion) is a depressant substance of the benzodiazepine class that produces anxiolytic, anticonvulsant, sedative, and amnesic effects when administered.[2]
An oral dose of 0.5mg triazolam roughly equals 10mg diazepam.[3]
Users should note that the sudden discontinuation of benzodiazepines can be potentially dangerous or life-threatening for individuals using regularly for extended periods of time, sometimes resulting in seizures or death.[4] It is highly recommended to taper one's dose by gradually lowering the amount taken each day for a prolonged period of time instead of stopping abruptly.[5]
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Triazolam was initially patented in 1970 and went on sale in the United States in 1982.[6] Its use at low doses has been deemed acceptable by the U.S. Food and Drug Administration (FDA) and several other countries.[7]
Triazolam issued nonmedically: recreational use wherein the drug is taken to achieve a high or continued long-term dosing against medical advice.[8] The drug is marketed in English-speaking countries under the brand names Apo-Triazo, Halcion, Hypam, and Trilam. Other (designer) names include 2'-chloroxanax, chloroxanax, triclazolam, and chlorotriazolam.
Chemistry
Triazolam is a drug of the benzodiazepine class. Benzodiazepine drugs contain a benzene ring fused to a diazepine ring, which is a seven membered ring with the two nitrogen constituents located at R1 and R4.
Triazolam has a molecular weight of 343.2 g/mol.
Pharmacology
Benzodiazepines produce a variety of effects by binding to the benzodiazepine receptor site and magnifying the efficiency and effects of the neurotransmitter gamma aminobutyric acid (GABA) by acting on its receptors.[9] As this site is the most prolific inhibitory receptor set within the brain, its modulation results in the sedating (or calming effects) of Triazolam on the nervous system.
The anticonvulsant properties of benzodiazepines may be, in part or entirely, due to binding to voltage-dependent sodium channels rather than benzodiazepine receptors.[10]
Triazolam is a triazolobenzodiazepine with hypnotic properties, advocated for use in acute or chronic insomnia, situational insomnia in hospitalised patients, and insomnia associated with other disease states. As triazolam has a relatively short half-life of about 2 to 3 hours in healthy subjects and has only 1 short acting active metabolite, alpha-hydroxytriazolam, it would seem more suitable as a hypnotic than longer acting drugs such as flurazepam, nitrazepam or flunitrazepam, particularly when residual sedative effects on the day after ingestion are undesirable.[11]
The bioavailability of triazolam after sublingual administration is increased by an average of 28% compared with oral administration of the same dose, possibly because first-pass extraction is bypassed. Clinical effects of triazolam may likewise be enhanced by sublingual dosage.[12] Unlike most other benzodiazepines, triazolam has a much higher intranasal bioavailability (98%) compared to its oral bioavailability (44%).[13]
Triazolam is a short-acting benzodiazepine, is lipophilic, and is metabolised hepatically via oxidative pathways. The main pharmacological effects of triazolam are the enhancement of the neurotransmitter GABA at the GABAA receptor.[14]
Subjective effects
The general head space of triazolam is described as one of intense sedation, relaxation, anxiety suppression and decreased inhibition similar to the headspace of higher doses of diazepam.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation - Triazolam can produce extreme sedation and often results in an overwhelmingly lethargic state. At higher levels, this causes users to suddenly feel as if they are extremely sleep deprived and have not slept for days, forcing them to sit down and generally feel as if they are constantly on the verge of passing out instead of engaging in physical activities. This sense of sleep deprivation increases proportional to dosage and eventually becomes powerful enough to force a person into complete unconsciousness.
- Dizziness
- Muscle relaxation
- Motor control loss
- Respiratory depression
- Seizure suppression
- Decreased heart rate
- Decreased blood pressure
- Perception of bodily heaviness - Triazolam is known to cause feelings of heaviness in the body. This effect can range from motor impairment and difficulty moving at lower doses to complete lethargy or inability to stand up or move at high doses.
- Physical euphoria - The euphoria felt on triazolam is significantly stronger than that felt on other benzodiazepines such as alprazolam.
- Dry mouth
Paradoxical effects 
- Paradoxical reactions to benzodiazepines such as increased seizures (in epileptics), aggression, increased anxiety, violent behavior, loss of impulse control, irritability and suicidal behavior sometimes occur (although they are rare in the general population, with an incidence rate below 1%).[15][16] These paradoxical effects occur with greater frequency in recreational abusers, individuals with mental disorders, children, and patients on high-dosage regimes.[17][18]
Cognitive effects 
-
The cognitive effects of triazolam can be broken down into several components which progressively intensify proportional to dosage. It contains a large number of typical depressant cognitive effects.
The most prominent of these cognitive effects generally include:
- Amnesia
- Cognitive euphoria - The cognitive euphoria felt on triazolam is significantly stronger than that felt on other benzodiazepines such as alprazolam.
- Anxiety suppression
- Thought deceleration
- Analysis suppression
- Disinhibition
- Compulsive redosing - Due to its short duration and delusions of sobriety, compulsive redosing has been reported.
- Emotion suppression - Although this compound primarily suppresses anxiety, it also dulls other emotions in a manner which is distinct but less intensive than that of antipsychotics.
- Delusions of sobriety - This is the false belief that one is perfectly sober despite obvious evidence to the contrary such as severe cognitive impairment and an inability to fully communicate with others. It most commonly occurs at heavy dosages.
- Memory suppression - Triazolam primarily suppresses short-term memory, resulting in forgetfulness, and/or disorganized behaviors.
- Confusion - At heavy doses, triazolam can cause confusion. This effect is a result of the drug suppressing basic cognitive functions at heavy doses, such as comprehension, memory, and reasoning skills.
- Motivation suppression - Due to triazolam's heavy sedation and lethargy, doing any type of activity that requires moving, or high amounts of effort may be difficult to do on this compound, especially at higher doses.
- Language suppression - Triazolam causes slurred speech and difficulty communicating words in a clear or coherent fashion.
- Dream potentiation - While most people report that benzodiazepines like triazolam tend to result in states of dreamless sleep, the opposite effect has also been reported, and seems to be particularly prevalent when a user is experiencing withdrawal from GABAergic substances (with alcohol being one notable example). The cause for these differences remain unclear at this point in time.
After effects 
-
- Rebound anxiety - Rebound anxiety is a commonly observed effect with anxiety relieving substances like benzodiazepines. It typically corresponds to the total duration spent under the substance's influence along with the total amount consumed in a given period, an effect which can easily lend itself to cycles of dependence and addiction.
- Residual sleepiness - While benzodiazepines can be used as an effective sleep-inducing aid, their effects may persist into the morning afterward, which may lead users to feeling "groggy" or "dull" for up to a few hours.
- Dream potentiation or Dream suppression
- Residual sleepiness
- Thought deceleration
- Thought disorganization
- Irritability
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Usage
Preparation methods
- Volumetric liquid dosing - If one's benzodiazepines are in powder form, they are unlikely to weigh out accurately without the most expensive of scales due to their extreme potency. To avoid this, one can dissolve the benzodiazepine volumetrically into a solution and dose it accurately based upon the methodological instructions linked within this tutorial here.
Toxicity and harm potential
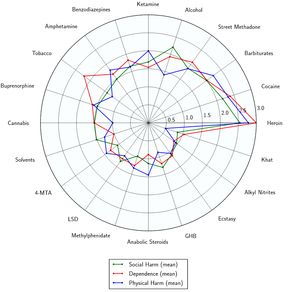
Triazolam likely has a low toxicity relative to dose.[2] However, it is potentially lethal when mixed with depressants like alcohol or opioids.
It is strongly recommended that one use harm reduction practices when using this drug.
Lethal dosage
The oral LD50 (lethal dose in 50% of the population) of triazolam is 1080 mg/kg in mice and over 7500 mg/kg in rats. [20]
Tolerance and addiction potential
Triazolam is extremely physically and psychologically addictive.
Tolerance will develop to the sedative-hypnotic effects within a couple of days of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. However, in certain cases, this may take significantly longer in a manner which is proportional to the duration and intensity of one's long-term usage.
Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few weeks or longer of steady dosing and may necessitate a gradual dose reduction. For more information on tapering from benzodiazepines in a controlled manner, please see this guide.
Benzodiazepine discontinuation is notoriously difficult; it is potentially life-threatening for individuals regularly using to discontinue use without tapering their dose over a period of weeks. There is an increased risk of hypertension, seizures, and death.[4] Drugs which lower the seizure threshold such as tramadol should be avoided during withdrawal.
Triazolam presents cross-tolerance with all benzodiazepines, meaning that after its consumption, all benzodiazepines will have a reduced effect.
Dangerous interactions
Although many drugs are safe on their own, they can become dangerous and even life-threatening when combined with other substances. The list below contains some common potentially dangerous combinations, but may not include all of them. Certain combinations may be safe in small doses of each but still, increase the potential risk of death. Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
- Depressants (1,4-Butanediol, 2-methyl-2-butanol, alcohol, barbiturates, GHB/GBL, methaqualone, opioids) - This combination can result in dangerous or even fatal levels of respiratory depression. These substances potentiate the muscle relaxation, sedation and amnesia caused by one another and can lead to unexpected loss of consciousness at high doses. There is also an increased risk of vomiting during unconsciousness and death from the resulting suffocation. If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Dissociatives - This combination can result in an increased risk of vomiting during unconsciousness and death from the resulting suffocation. If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants - It is dangerous to combine benzodiazepines with stimulants due to the risk of excessive intoxication. Stimulants decrease the sedative effect of benzodiazepines, which is the main factor most people consider when determining their level of intoxication. Once the stimulant wears off, the effects of benzodiazepines will be significantly increased, leading to intensified disinhibition as well as other effects. If combined, one should strictly limit themselves to only dosing a certain amount of benzodiazepines per hour. This combination can also potentially result in severe dehydration if hydration is not monitored.
- Ketoconazole and itraconazole have a profound effect on the pharmacokinetics of triazolam. This synergy can lead to a potentially dangerous enhancement of effects.[21]
Overdose
Benzodiazepine overdose may occur when a benzodiazepine is taken in large quantities or concurrently with other depressants. This is particularly dangerous with other GABAergic depressants such as barbiturates and alcohol since they work similarly, but bind to distinct allosteric sites on the GABAA receptor. Thus their effects potentiate one another. Benzodiazepines increase the frequency in which the chlorine ion pore opens on the GABAA receptor while barbiturates increase the duration in which they are open, meaning when both are consumed, the ion pore will open more frequently and stay open longer[22]. Benzodiazepine overdose is a medical emergency that may lead to a coma, permanent brain injury or death if not treated promptly and appropriately.
Symptoms of a benzodiazepine overdose may include severe thought deceleration, slurred speech, confusion, delusions, respiratory depression, coma or death. Benzodiazepine overdoses may be treated effectively in a hospital environment, with generally favorable outcomes. Benzodiazepine overdoses are sometimes treated with flumazenil, a GABAA antagonist[23]. However, care is primarily supportive in nature.
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
- International: Triazolam is a Schedule IV drug under the international Convention on Psychotropic Substances. [citation needed]
- Austria: Triazolam is legal for medical use under the AMG (Arzneimittelgesetz Österreich) and illegal when sold or possessed without a prescription under the SMG (Suchtmittelgesetz Österreich).[citation needed]
- Canada: Triazolam is a Schedule III controlled substance and is available by prescription only. [citation needed]
- Germany: Triazolam is controlled under Anlage III BtMG (Narcotics Act, Schedule III) as of August 1, 1986.[24] It can only be prescribed on a narcotic prescription form, except preparations which contain up to 0,25 mg triazolam in each dosage form.[25]
- United Kingdom: Triazolam is legal for medical use in the UK.[citation needed]
- United States: Triazolam is a Schedule IV drug under the Controlled Substances Act in the U.S. [citation needed]
See also
External links
- Triazolam (Wikipedia)
- Triazolam (Erowid Vault)
- Triazolam(Isomer Design)
- Triazolam (DrugBank)
- Triazolam (Drugs.com)
- Triazolam (Drugs-Forum)
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ 2.0 2.1 Benzodiazepine metabolism: an analytical perspective (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/18855614
- ↑ http://drugs.tripsit.me/triazolam
- ↑ 4.0 4.1 A fatal case of benzodiazepine withdrawal. (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/19465812
- ↑ Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain - Appendix B-6: Benzodiazepine Tapering | http://nationalpaincentre.mcmaster.ca/opioid/cgop_b_app_b06.html
- ↑ Shorter, Edward (2005). A Historical Dictionary of Psychiatry. Oxford University Press.
- ↑ Wishart, David (2006). "Triazolam". DrugBank. Retrieved 2006-03-23.
- ↑ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry. 66 Suppl 9: 31–41. PMID 16336040.
- ↑ Benzodiazepine interactions with GABA receptors (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/6147796
- ↑ Benzodiazepines, but not beta carbolines, limit high frequency repetitive firing of action potentials of spinal cord neurons in cell culture. (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/2450203
- ↑ https://www.sigmaaldrich.com/catalog/papers/6114852
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/3958225
- ↑ https://jpharmsci.org/article/S0022-3549(15)48710-6/pdf
- ↑ Oelschläger H. (1989-07-04). "Chemical and pharmacologic aspects of benzodiazepines". Schweiz Rundsch Med Prax. 78 (27–28): 766–72. PMID 2570451.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18922233 | Saïas T, Gallarda T | Paradoxical aggressive reactions to benzodiazepine use: a review
- ↑ Paton C | Benzodiazepines and disinhibition: a review | Psychiatr Bull R Coll Psychiatr | http://pb.rcpsych.org/cgi/reprint/26/12/460.pdf
- ↑ Bond AJ | Drug-induced behavioural disinhibition: incidence, mechanisms and therapeutic implications | CNS Drugs
- ↑ Drummer OH | Benzodiazepines—effects on human performance and behavior | Forensic Sci Rev
- ↑ Development of a rational scale to assess the harm of drugs of potential misuse (ScienceDirect) | http://www.sciencedirect.com/science/article/pii/S0140673607604644
- ↑ https://roempp.thieme.de/roempp4.0/do/data/RD-20-02690
- ↑ Varhe A, Olkkola KT, Neuvonen PJ (December 1994). "Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazole". Clin. Pharmacol. Ther. 56 (6 Pt 1): 601–7.
- ↑ Barbiturates and benzodiazepine effects | https://www.ncbi.nlm.nih.gov/pubmed/2471436
- ↑ Flumazenil, a benzodiazepine antagonist | https://www.ncbi.nlm.nih.gov/pubmed/8306565
- ↑ "Zweite Verordnung zur Änderung betäubungsmittelrechtlicher Vorschriften" (PDF) (in German). Bundesanzeiger Verlag. Retrieved December 26, 2019.
- ↑ "Anlage III BtMG" (in German). Bundesministerium der Justiz und für Verbraucherschutz. Retrieved December 26, 2019.
Halcion or triazolam half-life? 2-5 hours and I take all benzos on empty stomach ,
I have been using it Halcyon or triazolam 0.25 mg daily for insomnia. Algo2021 (talk) 01:41, 29 October 2021 (UTC)