Psilocin
| Summary sheet: Psilocin |
| Psilocin | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||
| Common names | Psilocin, Psilocine, Psilocyn, Psilotsin, 4-HO-DMT, 4-OH-DMT | ||||||||||||||||||||||||||||||||
| Substitutive name | 4-Hydroxy-N,N-dimethyltryptamine | ||||||||||||||||||||||||||||||||
| Systematic name | 3-[2-(Dimethylamino)ethyl]-1H-indol-4-ol | ||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||
| Psychoactive class | Psychedelic | ||||||||||||||||||||||||||||||||
| Chemical class | Tryptamine | ||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||
4-Hydroxy-N,N-dimethyltryptamine (also known as 4-HO-DMT, 4-OH-DMT, and psilocin) is a naturally-occurring psychedelic substance of the tryptamine class. Psilocin is the primary psychoactive constituent in certain species of mushrooms, and as a closely related structural analog of the powerful visionary entheogen DMT (also known as N,N-dimethyltryptamine).
Psilocin was first isolated and named by Albert Hofmann in 1958. Its psychoactivity is thought to emerge from the close chemical similarities with the neurotransmitter serotonin, which enables it to interact with a range of serotonin receptor sites throughout the brain that are integral for sensory and cognitive processes.
Notably, while psilocin naturally co-occurs with psilocybin in significant amounts of most psilocybin-containing mushrooms, it is only ever rarely encountered in its synthetic form. Anecdotal reports describe pure psilocin as a more lucid and aggressive version of psilocybin mushrooms.
Unlike other highly prohibited substances, psilocin is not considered to be addictive or physiologically toxic.[1][2] Nevertheless, adverse psychological reactions such as severe anxiety, paranoia and psychosis are always possible, particularly among those predisposed to mental illness.[3] It is highly advised to use harm reduction practices if using this substance.
History and culture
This History and culture section is a stub. As a result, it may contain incomplete or wrong information. You can help by expanding it. |
Psilocin and its phosphorylated inactive precursor (i.e. prodrug) psilocybin were first isolated and named in 1958 by Swiss chemist Albert Hofmann. Hofmann obtained the chemicals from laboratory-grown specimens of the entheogenic mushroom Psilocybe mexicana before proceeding to find their synthetic routes.[4]
Chemistry
Psilocin, or 4-HO-DMT, is an organic indole alkaloid molecule of the tryptamine class of chemicals. Tryptamines share a core structure comprised of a bicyclic indole heterocycle attached at R3 to an amino group via an ethyl side chain. 4-HO-DMT is substituted at R4 of its indole heterocycle with an hydroxyl (-OH) functional group; it also contains two methyl groups CH3- bound to the terminal amine RN of the ethyl side chain. This makes psilocin the 4-hydroxy structural analog of DMT, and dephosphorylated analog of psilocybin.
Psilocin can be obtained by dephosphorylation of natural psilocybin under strongly acidic or under alkaline conditions via hydrolysis,[citation needed] which is how it becomes metabolically active in the human body as well.[citation needed]
In terms of its physical properties, 4-HO-DMT is relatively unstable in solution due to its phenolic hydroxy (-OH) group. In the presence of oxygen it readily forms bluish and dark black degradation products.[citation needed] For this reason it is recommended to store it in optimal chemical storage conditions (i.e. cool, dry, away from light) to avoid excessive degradation.
Pharmacology
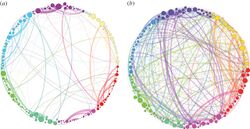
Psilocin is the pharmacologically active agent in the body after ingestion of psilocybin or some species of psychedelic mushrooms.
Psilocybin is rapidly dephosphorylated in the body to psilocin which acts as a 5-HT2A, 5-HT2C and 5-HT1A agonist or partial agonist. Psilocin exhibits functional selectivity in that it activates phospholipase A2 instead of activating phospholipase C as the endogenous ligand serotonin does. Psilocin is structurally similar to serotonin (5-HT),[6] differing only by the hydroxyl group being on the 4-position rather than the 5 and the dimethyl groups on the nitrogen. Its effects are thought to come from its agonist activity at 5-HT2A serotonin receptors in the prefrontal cortex.
Psilocin's psychedelic effects are believed to come from its interactions at the 5-HT2A receptor as a partial agonist. However, the role of these interactions and how they result in the psychedelic experience remains the subject of ongoing scientific investigation.
In contrast to LSD, this compound has no significant effect on dopamine receptors and only affects the noradrenergic system at very high dosages.[7]
Subjective effects
Anecdotal reports characterize the effects of psilocin as powerful and visionary, with a deep, all-encompassing headspace, immersive visuals with high-level geometry, and a rapid challenging come up that is both reportedly more lucid and anxiety-provoking than orally ingested psilocybin mushrooms; this may make the experience overly intense for those who are not experienced with psychedelics.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects 
-
- Sedation - In terms of its effects on the physical energy levels of the tripper, psilocin is considered by most to be relaxing, stoning and mildly sedating. This sense of sedation is often accompanied by compulsive yawning.
- Perception of bodily heaviness - This effect corresponds with the general sense of sedation and relaxation that characterizes psilocin experiences, this manifests as a bodily heaviness that discourages movement but is typically only prominent during the first half of the trip. This particular physical effect seems to be more commonly experienced and pronounced with certain “woodlover” species of mushrooms such as Psilocybe azurescens.
- Spontaneous physical sensations - The "body high" of psilocin can be described as a pleasurable, soft and all-encompassing tingling sensation or glow. This maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached. Once the peak of the experience or sensation is reached it can feel incredibly euphoric and tranquil or heavy and immobilizing depending on the dose.
- Physical euphoria - It should be noted that this effect is not as reliably induceable for psychedelics like psilocin than it is with other substance classes like stimulants or entactogens, and can just as easily manifest as physical discomfort for no apparent reason.
- Changes in felt bodily form - This effect is often accompanied by a sense of warmth or unity and usually occurs around the peak of the experience or directly after. Users can feel as if they are physically part of or conjoined with other objects. This is usually reported as feeling comfortable in its sensations and even peaceful.
- Tactile enhancement - This effect is less prominent than with that of LSD or 2C-B but is still present and unique in its character. It is repeatedly described as feeling very primitive in its nature often times with the small hairs on the users arms or legs feeling slightly itchy or even ticklish against the skin.
- Changes in felt gravity
- Nausea - This effect can be greatly lessened or even completely avoided if the individual has an empty stomach prior to ingestion. It is often recommended that one either refrain from eating for approximately 6 to 8 hours beforehand, or eat a light meal 3 to 4 hours before if they are feeling physically fatigued. In the rare circumstances that pure psilocin is ingested this effect is considered to be much less prominent than it is with psilocybin mushrooms.
- Excessive yawning - This effect seems to be uniquely pronounced among psilocin and related tryptamines. It can occur to a lesser degree on LSD and very rarely on psychedelic phenethylamines like mescaline. It typically occurs in combination with watery eyes.
- Brain zaps - Although this effect is very rare, it can still occur for those susceptible to it. This component is however much less common and intense than it is with serotonin releasing agents such as MDMA.
- Watery eyes
- Muscle contractions
- Olfactory hallucination
- Frequent urination
- Pupil dilation
- Runny nose
- Increased salivation
- Seizure - This is a rare effect but can happen in those predisposed to them, especially while in physically taxing conditions such as being dehydrated, undernourished, or fatigued.
- Sedation - In terms of its effects on the physical energy levels of the tripper, psilocin is considered by most to be relaxing, stoning and mildly sedating. This sense of sedation is often accompanied by compulsive yawning.
Visual effects 
-
Enhancements
- Colour enhancement - In comparison to other psychedelics, this effect may appear to be more saturated.
- Visual acuity enhancement - This effect typically occurs at lower to medium doses and becomes increasingly suppressed at higher doses.
- Magnification
- Pattern recognition enhancement
Distortions
- Drifting (melting, flowing, breathing and morphing) - In comparison to other psychedelics, this effect can be described as highly detailed, realistic, slow and smooth in motion and static in appearance.
- Colour shifting
- Colour tinting
- Visual haze
- Diffraction
- Tracers
- After images
- Symmetrical texture repetition
- Perspective distortions
- Depth perception distortions
- Environmental orbism
- Scenery slicing
- Environmental patterning
Geometry
The visual geometry present on this substance can be described as more similar in appearance to that of 4-AcO-DMT, ayahuasca and 2C-E than LSD or 2C-B. It can be comprehensively described through its variations as intricate in complexity, abstract in form, organic in feel, structured in organization, brightly lit, and multicoloured in scheme, glossy in shading, soft in its edges, large in size, slow in speed, smooth in motion, rounded in its corners, non-immersive in-depth and consistent in intensity. It has a very "organic" feel and at higher dosages is significantly more likely to result in states of Level 8B visual geometry over level 8A.
Hallucinatory states
Psilocin and its various other forms produce a full range of high level hallucinatory states in a fashion that is more consistent and reproducible than that of many other commonly used psychedelics. These effects generally include:
- Machinescapes
- Transformations
- Internal hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - This effect is very consistent in dark environments at appropriately high dosages. They can be comprehensively described through their variations as lucid in believability, interactive in style, new experiences in content, autonomous in controllability, geometry-based in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical, or transcendental nature in their overall theme.
- External hallucination (autonomous entities; settings, sceneries, and landscapes; perspective hallucinations and scenarios and plots) - These are more common within dark environments and can be comprehensively described through their variations as lucid in believability, interactive in style, new experiences in content, autonomous in controllability, geometry-based in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
- Shadow people
Cognitive effects 
-
The cognitive effects of psilocin are described by many as extremely relaxing, profound and stoning in style when compared to other commonly used psychedelics such as LSD or 2C-B which tend to be energetic and stimulating, it is also regarded as being notably more lucid than psilocybin mushrooms but not as clearheaded as DMT. It contains a large number of both typical and unique psychedelic cognitive effects.
The most prominent of these typical effects generally include:
- Enhancement and suppression cycles - This can be described as constant waves of extremely stimulated and profound thinking which are spontaneously surpassed in a cyclic fashion by waves of general thought suppression and mental intoxication. These two states seem to switch between each other in a consistent loop once every 20 to 60 minutes.
- Emotionality enhancement - This effect can be described as being more prominent, consistent and profound when compared to other traditional psychedelics such as mescaline or LSD. This can lead to strong feelings of compassion, urgency and even completely sporadic moments of intense emotional significance that can also be periodically affected by enhancement and suppression cycles.
- Empathy, affection, and sociability enhancement - This effect differs from MDMA and other entactogens in that it isn't as central to the experience, feels less forced and more natural and is experienced at a less consistent rate. The sociability enhancement in particular only occurs rarely and it appears to be more emotional.
- Simultaneous emotions
- Language suppression - This effect can be described as a perceived inability or general unwillingness to talk aloud despite feeling perfectly capable of formulating coherent thoughts within one's internal narrative. It is much more common among inexperienced users.
- Autonomous voice communication
- Cognitive euphoria
- Analysis enhancement - This effect is consistent in its manifestation and outrospection dominant.
- Conceptual thinking
- Personal bias suppression
- Novelty enhancement
- Immersion enhancement
- Creativity enhancement
- Feelings of impending doom - This effect is usually only experienced during the come up phase but typically completely passes or subsides once the primary effects begin. It should be noted that this effect is relatively consistent and normal for psilocin and related tryptamines which is why a positive and well-informed mindset is key. Less regularly this aspect can also occur during the peak but will most often be met after with sensations of euphoria or rejuvenation.
- Catharsis - While this component can occur spontaneously, it typically follows a difficult phase of the experience, if not the entirety of the experience itself.
- Rejuvenation- While this component can occur spontaneously at any point, it typically follows a difficult phase of the experience, if not the entire experience itself. It is however almost always felt during the offset of a psilocin trip and tends to slowly transition into the after effects which are generally described as positive. These positive or mindful after effects are sometimes referred to as an "afterglow" and is both common and consistent for psilocin and related tryptamines.
- Memory suppression
- Addiction suppression[8]
- Thought connectivity
- Thought deceleration
- Thought organization
- Confusion - This effect is commonly reported to occur at a higher rate than with other psychedelics such as LSD or mescaline. It is more commonly observed in users who are generally inexperienced with psychedelics.
- Delusion
- Déjà vu
- Increased music appreciation
- Increased sense of humor
- Mindfulness
- Ego replacement - Although this effect is rare and more likely to occur with certain psychedelics like DMT or ayahuasca, it can still occur with high dosages.
- Personality regression - While this effect is not typically observed, it can still spontaneously manifest and is thought to depend primarily on the user's set and setting.
- Time distortion
Auditory effects 
Multi-sensory effects 
-
- Synaesthesia - In its fullest manifestation, this is a very rare and non-reproducible effect. Increasing the dosage can increase the likelihood of this occurring, but seems to only be a prominent part of the experience among those who are already predisposed to synaesthetic states.
- Dosage independent intensity[citation needed]
Transpersonal effects 
- Anecdotally, components such as these are generally considered to be most consistent with the naturally occurring entheogenic tryptamines such as ayahuasca, ibogaine and psilocybin/psilocin.
Combination effects
- Cannabis - Cannabis intensifies the visual, sensory and cognitive effects of psilocin greatly. This should be used with extreme caution, especially if one is not experienced with psychedelics. This interaction can also amplify the anxiety, confusion and the psychosis risk of cannabis significantly.
- Dissociatives - Psilocin enhances the the geometry, euphoria, dissociation and hallucinatory effects of dissociatives. Dissociative-induced holes, spaces, and voids while under the influence of psilocin can result in significantly more vivid visuals than dissociatives alone present, along with more intense internal hallucinations, confusion, nausea, delusions and chances of a psychotic reaction.
- MDMA - Psilocin strongly amplifies the visual, physical and cognitive effects of MDMA. The synergy between these substances is unpredictable, and it is best to start with lower dosages than one would take for both substances individually. The toxicity of this combination is unknown, although there is some evidence that suggests this may increase the the neurotoxic effects of MDMA.[9][10][11]
- Alcohol - This combination is typically advised against due to alcohol’s ability to cause dehydration, nausea, and physical fatigue which can negatively affect the experience if taken in moderate to high dosages. This combination is, however, considered to be reasonably safe in low doses and when used responsibly, this can often take the edge off the experience as well as dull its psychedelic effects in a fashion somewhat similar to benzodiazepines.
- Benzodiazepines - Depending on the dosage, benzodiazepines can slightly to completely reduce the intensity of the cognitive, physical and visual effects of a psilocin experience. They can be very efficient at largely stopping or mitigating a bad trip at the cost of amnesia and reduced intensity. Caution is advised when obtaining them for this purpose due to their very high addiction and abuse potential.
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
Additional experience reports can be found here:
Research
Antidepressant effects
While further research is needed to establish the utility of psilocybin and other psychedelics in treating depression, a pilot study has observed significantly decreased depression scores in terminal cancer patients six months after treatment with psilocybin.[12] An open-label study was carried out in 2016 in the UK to investigate the feasibility, safety and efficacy of psilocybin in treating patients with unipolar treatment-resistant depression with promising results; although the study was small and involved only twelve patients, seven of those patients met formal criteria for remission one week following psilocybin treatment and five of those were still in remission from their depression at three months.[13]
The mechanism behind this is not known as of yet, but researchers have suggested that psilocin's deactivation of the medial prefrontal cortex[14] (mPFC) may be relevant to its antidepressant effects, as the mPFC is known to be elevated in depression and normalized after effective treatment.[14] mPFC hyperactivity has been associated with trait rumination.[15] Another possible factor to psilocybin's potential against depression may be that depressed patients with high levels of dysfunctional attitudes were found to have low levels of 5-HT(2A) agonism.[16][17]
Toxicity and harm potential
Psilocin is non-addictive, is not known to cause brain damage, and has an extremely low toxicity relative to dose. Similar to other psychedelic drugs, there are relatively few physical side effects associated with acute psilocin exposure. Various studies have shown that in reasonable doses in a careful context, it presents little to no negative cognitive, psychiatric or toxic physical consequences.[citation needed]
Lethal dosage
The toxicity of psilocybin and psilocin is extremely low. In rats, the median lethal dose (LD50) of psilocybin when administered orally is 280 milligrams per kilogram (mg/kg). Psilocybin comprises approximately 1% of the weight of Psilocybe cubensis mushrooms and so nearly 1.7 kilograms (3.7 lb) of dried mushrooms or 17 kilograms (37 lb) of fresh mushrooms would be required for a 60 kilogram (130 lb) person to reach the 280 mg/kg LD50 value of rats. Based on the results of animal studies, the lethal dose of psilocybin has been extrapolated to be 6 grams, 1000 times greater than the effective dose of 6 milligrams.[citation needed]
Despite its lack of physical toxicity, however, it is still strongly recommended that one use harm reduction practices if choosing to use this substance.
Tolerance and addiction potential
Psilocin is not habit-forming, and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of psilocin is built almost immediately after ingestion. After that, it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). Psilocin presents cross-tolerance with all psychedelics, meaning that after the consumption of psilocin all psychedelics will have a reduced effect.
Legal status
Psilocin is a Schedule I drug under the Convention on Psychotropic Substances, meaning the possession and sale of it (including psilocin and psilocybin-containing mushrooms) is prohibited in most countries.[18]
- Belgium: Possession and sale of mushrooms have been illegal since 1988.[citation needed]
- Brazil: Possession, production and sale is illegal as it is listed on Portaria SVS/MS nº 344[19], but mushrooms fall under religious use laws.[citation needed]
- British Virgin Isles: The sale of mushrooms is illegal, but possession and consumption is legal.[citation needed]
- Bulgaria: The sale of mushrooms is illegal, but possession and consumption is legal.[citation needed]
- Canada: Psilocybin and psilocin are illegal to possess, obtain or produce without a prescription or license as they are Schedule III under the Controlled Drugs and Substances Act.[20]
- Cyprus The possession, sale and consumption of mushrooms is illegal.[citation needed]
- Czech Republic: The distribution (including sale) of mushrooms is illegal, but consumption is legal. The possession of over 40 hallucinogenic caps is considered a crime if they contain more than 50mg of psilocin or the corresponding amount of psilocybin. The possession of more than 40g of hallucinogenic mycelium is considered a crime. If these limits are not exceeded, the act is considered a minor offence and a fine of up to 15 thousand CZK may be imposed.[citation needed]
- Denmark: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- Finland: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- Germany: Psilocin is controlled under Anlage I BtMG[21] (Narcotics Act, Schedule I), former: Opiumgesetz (Opium Act) as of February 25, 1967.[22] It is illegal to manufacture, possess, import, export, buy, sell, procure or dispense it without a license.[23]
- Greece: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- Ireland: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- Japan: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- Latvia: Hallucinogenic mushrooms, psilocin and psilocibyn are Schedule I controlled substances.[24]
- Mexico: The possession, growth, sale and consumption of mushrooms is illegal. Rules are relaxed regarding religious use however.[citation needed]
- The Netherlands: The possession, growth, sale and consumption of mushrooms is illegal. However, due to a legal loophole, psilocybin truffles can be legally possessed, grown, sold and consumed.
- New Zealand: Psilocybin is Class A.[citation needed]
- Norway: Possession, growth, sale and consumption of mushrooms is illegal. Spores, even though not containing psilocybin, are also illegal.[citation needed]
- Switzerland: Psilocin is a controlled substance specifically named under Verzeichnis D. Mushrooms of the species Conocybe, Panaeolus, Psilocybe and Stropharia are also controlled under Verzechnis D.[25]
- Turkey: The possession, growth, sale and consumption of mushrooms is illegal.[citation needed]
- United Kingdom: According to the 2005 Drugs Act, fresh and prepared psilocybin mushrooms are Class A.[26]
- United States: Psilocybin and psilocin are illegal Schedule I drugs.[27]
See also
- Responsible use
- Entheogens
- Psychedelics
- Tryptamines
- Psilocybin
- Psilacetin (4-AcO-DMT)
- Metocin (4-HO-MET)
- DMT
External links
Discussion
- The Big & Dandy Psilocybin Mushrooms Thread (Bluelight)
- Psilocybin mushrooms (Disregard Everything I Say)
- World Wide Distribution of Magic Mushrooms
References
- ↑ Lüscher, Christian; Ungless, Mark A. (2006). "The Mechanistic Classification of Addictive Drugs". PLOS Medicine. 3 (11). doi:10.1371/journal.pmed.0030437. eISSN 1549-1676. ISSN 1549-1277. OCLC 54674092. PMC 1635740
 . PMID 17105338.
. PMID 17105338.
- ↑ Nichols, David E. (2016). Barker, Eric L., ed. "Psychedelics". Pharmacological Reviews. 68 (2): 264–355. doi:10.1124/pr.115.011478. eISSN 1521-0081. ISSN 0031-6997. OCLC 00824083. PMC 4813425
 . PMID 26841800.
. PMID 26841800.
- ↑ Strassmann, Rick (1984). "Adverse reactions to psychedelic drugs. A review of the literature". Journal of Nervous and Mental Disease. 172 (10): 577–595. doi:10.1097/00005053-198410000-00001. ISSN 0022-3018. OCLC 1754691. PMID 6384428.
- ↑ Hofmann, A.; Heim, R.; Brack, A.; Kobel, H.; Frey, A.; Ott, H.; Petrzilka, T.; Troxler, F. (1959). "Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen". Helvetica Chimica Acta (in German). 42 (5): 1557–1572. doi:10.1002/hlca.19590420518. ISSN 0018-019X. OCLC 1108916122.
- ↑ Petri, G.; Expert, P.; Turkheimer, F.; Nutt, D.; Hellyer, P. J.; Vaccarino, F. (2014). "Homological scaffolds of brain functional networks". Journal of the Royal Society Interface. 11 (101): 14–18. doi:10.1098/rsif.2014.0873. eISSN 1742-5662. ISSN 1742-5689. OCLC 711051718. PMC 4223908
 . PMID 25401177.
. PMID 25401177.
- ↑ Diaz, Jaime (1996). How Drugs Influence Behavior: A Neurobehavioral Approach. Englewood Cliffs: Prentice Hall. ISBN 9780023287640.
- ↑ Lisa Jerome (2007). "Psilocybin: Investigator's Brochure" (PDF). Multidisciplinary Association for Psychedelic Studies (MAPS).
- ↑ Johnson, M. W.; Garcia-Romeu, A.; Cosimano, M. P.; Griffiths, R. R. (2014). "Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction". Journal of Psychopharmacology. 28 (11): 983–992. doi:10.1177/0269881114548296. eISSN 1461-7285. ISSN 0269-8811. OCLC 19962867. PMC 4286320
 . PMID 25213996.
. PMID 25213996.
- ↑ Armstrong, B. D.; Paik, E.; Chhith, S.; Lelievre, V.; Waschek, J. A.; Howard, S. G. (2004). "Potentiation of (DL)‐3,4‐methylenedioxymethamphetamine (MDMA)‐induced toxicity by the serotonin 2A receptior partial agonist d‐lysergic acid diethylamide (LSD), and the protection of same by the serotonin 2A/2C receptor antagonist MDL 11,939". Neuroscience Research Communications. 35 (2): 83–95. doi:10.1002/nrc.20023. eISSN 1520-6769.
- ↑ Gudelsky, Gary A.; Yamamoto, Bryan; Nash, J. Frank (1994). "Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists". European Journal of Pharmacology. 264 (3): 325–330. doi:10.1016/0014-2999(94)90669-6. eISSN 1879-0712. ISSN 0014-2999. OCLC 01568459.
- ↑ Capela, J. P.; Fernandes, E.; Remião, F.; Bastos, M. L.; Meisel, A.; Carvalho, F. (2007). "Ecstasy induces apoptosis via 5-HT2A-receptor stimulation in cortical neurons". NeuroToxicology. 28 (4): 868–875. doi:10.1016/j.neuro.2007.04.005. ISSN 0161-813X. OCLC 47153737. PMID 17572501.
- ↑ Grob, C. S.; Danforth, A. L.; Chopra, G. S.; Hagerty, M.; McKay, C. R.; Halberstadt, A. L.; Greer, G. R. (2011). "Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer". Archives of General Psychiatry. 68 (1): 71–78. doi:10.1001/archgenpsychiatry.2010.116. eISSN 1538-3636. ISSN 2168-622X. PMID 20819978.
- ↑ Carhart-Harris, R. L.; Bolstridge, M.; Rucker, J.; Day, C. M.; Erritzoe, D.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; Rickard, J. A.; Forbes, B.; Feilding, A.; Taylor, D.; Curran, H. V.; Nutt, D. J. "Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study". The Lancet. Psychiatry. 3 (7): 619–627. doi:10.1007/s00213-017-4771-x. ISSN 2215-0374. OCLC 1091418082. PMC 5813086
 . PMID 29119217.
. PMID 29119217.
- ↑ 14.0 14.1 Carhart-Harris, R. L.; Erritzoe, D.; Williams, T.; Stone, J. M.; Reed, L. J.; Colasanti, A.; Tyacke, R. J.; Leech, R.; Malizia, A. L.; Murphy, K.; Hobden, P.; Evans, J.; Feilding, A.; Wise, R. G.; Nutt, D. J. (2012). "Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin". Proceedings of the National Academy of Sciences. 109 (6): 2138–2143. doi:10.1073/pnas.1119598109. eISSN 1091-6490. ISSN 0027-8424. OCLC 43473694. PMC 3277566
 . PMID 22308440.
. PMID 22308440.
- ↑ Farb, N. A. S.; Anderson, A. K.; Bloch, R. T.; Segal, Z. V. (2011). "Mood Linked Responses in Medial Prefrontal Cortex Predict Relapse in Patients with Recurrent Unipolar Depression". Biological Psychiatry. 70 (4): 366–372. doi:10.1016/j.biopsych.2011.03.009. eISSN 1873-2402. ISSN 0006-3223. OCLC 424038458. PMC 3145008
 . PMID 21531382.
. PMID 21531382.
- ↑ Bhagwagar, Z.; Hinz, R.; Taylor, M.; Fancy, S.; Cowen, P.; Grasby, P. (2006). "Increased 5-HT2A receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [11C]MDL 100,907". American Journal of Psychiatry. 163 (9): 1580–1587. doi:10.1176/ajp.2006.163.9.1580. eISSN 1535-7228. ISSN 0002-953X. OCLC 1480183. PMID 16946184.
- ↑ Meyer, J. H.; McMain, S.; Kennedy, S. H.; Korman, L.; Brown, G. M.; DaSilva, J. N.; Wilson, A. A.; Blak, T.; Eynan-Harvey, R.; Goulding, V. S.; Houle, S.; Links, P. (2003). "Dysfunctional Attitudes and 5-HT2 Receptors During Depression and Self-Harm". American Journal of Psychiatry. 160 (1): 90–99. doi:10.1176/appi.ajp.160.1.90. eISSN 1535-7228. ISSN 0002-953X. OCLC 1480183. PMID 12505806.
- ↑ "List of Psychotropic Substances under International Control (Green List)" (PDF). International Narcotics Control Board (INCB). Archived from the original (PDF) on October 2, 2017.
- ↑ "RESOLUÇÃO DA DIRETORIA COLEGIADA - RDC N° 130, DE 2 DE DEZEMBRO DE 2016" (in Portuguese). Agência Nacional de Vigilância Sanitária (Anvisa) [National Sanitary Surveillance Agency]. December 5, 2016. Archived from the original on September 24, 2020.
- ↑ "Schedule III". Controlled Drugs and Substances Act (S.C. 1996, c. 19). Government of Canada. Retrieved January 1, 2020.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln: Anlage I" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Vierte Verordnung über die den Betäubungsmitteln gleichgestellten Stoffe" (PDF). Bundesgesetzblatt Teil I: 1967 Nr. 10 (in German). Bundesanzeiger Verlag. February 24, 1967. p. 197. ISSN 0341-1095.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln: § 29" (in German). Bundesamt für Justiz [Federal Office of Justice]. Retrieved December 10, 2019.
- ↑ "Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem" (in Latvian). VSIA Latvijas Vēstnesis. November 10, 2005. Retrieved January 1, 2020.
- ↑ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Bundeskanzlei [Federal Chancellery of Switzerland]. Retrieved January 1, 2020.
- ↑ "Drugs Act 2005". UK Government. Retrieved August 24, 2020.
- ↑ "Title 21 - Food And Drugs: Chapter 13 - Drug Abuse Prevention And Control: Subchapter I - Control And Enforcement". Controlled Substances Act. U.S. Food and Drug Administration. Archived from the original on August 31, 2016.